JEE Exam > JEE Questions > Conversion of benzene diazonium chloride to c...
Start Learning for Free
Conversion of benzene diazonium chloride to chloro benzene is an example of which of the following reactions ?
- a)Claisen
- b)Friedel-craft
- c)Sandmeyer
- d)Wurtz
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Conversion of benzene diazonium chloride to chloro benzene is an examp...
Sandmeyer reaction involves the reaction of benzene diazonium salts with the solution of copper halide, and hydrochloric acid leads to the formation of halogen substituted benzene. The conversion of benzene diazonium salt to chlorobenzene is shown below.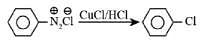
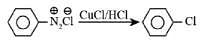
Most Upvoted Answer
Conversion of benzene diazonium chloride to chloro benzene is an examp...
The conversion of benzene diazonium chloride to chlorobenzene is an example of the Sandmeyer reaction.
Sandmeyer Reaction:
The Sandmeyer reaction is a chemical reaction used to convert an aromatic diazonium salt to a new functional group. It involves the substitution of the diazonium group with another functional group, typically through the use of a copper(I) salt as a catalyst. The reaction is named after the Swiss chemist Traugott Sandmeyer, who first described it in the late 19th century.
Reaction Mechanism:
The Sandmeyer reaction proceeds through a series of steps:
1. Diazonium ion formation: The benzene diazonium chloride (C6H5N2Cl) reacts with hydrochloric acid (HCl) to form the diazonium ion (C6H5N2+).
2. Copper(I) salt formation: Copper(I) chloride (CuCl) is added as a catalyst to facilitate the reaction.
3. Nitrogen gas evolution: The diazonium ion loses nitrogen gas (N2) to form a carbocation intermediate.
4. Substitution: The carbocation intermediate reacts with a nucleophile (chloride ion, Cl-) to form the desired product, chlorobenzene (C6H5Cl).
Significance of the Sandmeyer Reaction:
The Sandmeyer reaction is an important tool in synthetic organic chemistry as it allows for the introduction of a wide range of functional groups onto aromatic rings. It is particularly useful for the preparation of aryl halides, which are important intermediates in the synthesis of various organic compounds.
Other Reactions:
- Claisen reaction: The Claisen reaction is a reaction between an ester and a strong base to form a β-keto ester.
- Friedel-Crafts reaction: The Friedel-Crafts reaction is a reaction between an aromatic compound and an alkyl halide or acyl halide to form a substituted aromatic compound.
- Wurtz reaction: The Wurtz reaction is a reaction between alkyl halides and metallic sodium to form a higher hydrocarbon.
Therefore, the conversion of benzene diazonium chloride to chlorobenzene is an example of the Sandmeyer reaction (option C).
Sandmeyer Reaction:
The Sandmeyer reaction is a chemical reaction used to convert an aromatic diazonium salt to a new functional group. It involves the substitution of the diazonium group with another functional group, typically through the use of a copper(I) salt as a catalyst. The reaction is named after the Swiss chemist Traugott Sandmeyer, who first described it in the late 19th century.
Reaction Mechanism:
The Sandmeyer reaction proceeds through a series of steps:
1. Diazonium ion formation: The benzene diazonium chloride (C6H5N2Cl) reacts with hydrochloric acid (HCl) to form the diazonium ion (C6H5N2+).
2. Copper(I) salt formation: Copper(I) chloride (CuCl) is added as a catalyst to facilitate the reaction.
3. Nitrogen gas evolution: The diazonium ion loses nitrogen gas (N2) to form a carbocation intermediate.
4. Substitution: The carbocation intermediate reacts with a nucleophile (chloride ion, Cl-) to form the desired product, chlorobenzene (C6H5Cl).
Significance of the Sandmeyer Reaction:
The Sandmeyer reaction is an important tool in synthetic organic chemistry as it allows for the introduction of a wide range of functional groups onto aromatic rings. It is particularly useful for the preparation of aryl halides, which are important intermediates in the synthesis of various organic compounds.
Other Reactions:
- Claisen reaction: The Claisen reaction is a reaction between an ester and a strong base to form a β-keto ester.
- Friedel-Crafts reaction: The Friedel-Crafts reaction is a reaction between an aromatic compound and an alkyl halide or acyl halide to form a substituted aromatic compound.
- Wurtz reaction: The Wurtz reaction is a reaction between alkyl halides and metallic sodium to form a higher hydrocarbon.
Therefore, the conversion of benzene diazonium chloride to chlorobenzene is an example of the Sandmeyer reaction (option C).
Attention JEE Students!
To make sure you are not studying endlessly, EduRev has designed JEE study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in JEE.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Conversion of benzene diazonium chloride to chloro benzene is an example of which of the following reactions ?a)Claisenb)Friedel-craftc)Sandmeyerd)WurtzCorrect answer is option 'C'. Can you explain this answer?
Question Description
Conversion of benzene diazonium chloride to chloro benzene is an example of which of the following reactions ?a)Claisenb)Friedel-craftc)Sandmeyerd)WurtzCorrect answer is option 'C'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Conversion of benzene diazonium chloride to chloro benzene is an example of which of the following reactions ?a)Claisenb)Friedel-craftc)Sandmeyerd)WurtzCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Conversion of benzene diazonium chloride to chloro benzene is an example of which of the following reactions ?a)Claisenb)Friedel-craftc)Sandmeyerd)WurtzCorrect answer is option 'C'. Can you explain this answer?.
Conversion of benzene diazonium chloride to chloro benzene is an example of which of the following reactions ?a)Claisenb)Friedel-craftc)Sandmeyerd)WurtzCorrect answer is option 'C'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Conversion of benzene diazonium chloride to chloro benzene is an example of which of the following reactions ?a)Claisenb)Friedel-craftc)Sandmeyerd)WurtzCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Conversion of benzene diazonium chloride to chloro benzene is an example of which of the following reactions ?a)Claisenb)Friedel-craftc)Sandmeyerd)WurtzCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Conversion of benzene diazonium chloride to chloro benzene is an example of which of the following reactions ?a)Claisenb)Friedel-craftc)Sandmeyerd)WurtzCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Conversion of benzene diazonium chloride to chloro benzene is an example of which of the following reactions ?a)Claisenb)Friedel-craftc)Sandmeyerd)WurtzCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Conversion of benzene diazonium chloride to chloro benzene is an example of which of the following reactions ?a)Claisenb)Friedel-craftc)Sandmeyerd)WurtzCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Conversion of benzene diazonium chloride to chloro benzene is an example of which of the following reactions ?a)Claisenb)Friedel-craftc)Sandmeyerd)WurtzCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Conversion of benzene diazonium chloride to chloro benzene is an example of which of the following reactions ?a)Claisenb)Friedel-craftc)Sandmeyerd)WurtzCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Conversion of benzene diazonium chloride to chloro benzene is an example of which of the following reactions ?a)Claisenb)Friedel-craftc)Sandmeyerd)WurtzCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























