Class 12 Exam > Class 12 Questions > Benzoic acid from methyl benzene?
Start Learning for Free
Benzoic acid from methyl benzene?
Most Upvoted Answer
Benzoic acid from methyl benzene?
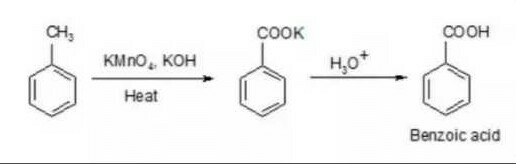
Community Answer
Benzoic acid from methyl benzene?
**Benzoic Acid from Methyl Benzene: A Detailed Explanation**
**Introduction:**
The conversion of methyl benzene (toluene) to benzoic acid involves a series of chemical reactions that change the structure of the molecule, leading to the formation of benzoic acid. This process typically involves oxidation and subsequent acidification of the methyl group on the benzene ring.
**Step 1: Oxidation of Methyl Benzene**
The first step in the conversion of methyl benzene to benzoic acid is the oxidation of the methyl group to a carboxylic acid group. This can be achieved through a variety of oxidation methods, but one commonly used approach is the reaction with a strong oxidizing agent such as potassium permanganate (KMnO4) or chromic acid (H2CrO4). The oxidation of the methyl group results in the formation of a carboxylic acid group, giving rise to the intermediate molecule, benzyl alcohol.
**Step 2: Oxidation of Benzyl Alcohol**
The second step involves the further oxidation of benzyl alcohol to benzoic acid. This step is typically carried out by using a strong oxidizing agent such as potassium permanganate (KMnO4). The oxidation of benzyl alcohol leads to the formation of benzoic acid.
**Step 3: Acidification of the Intermediate**
The final step is the acidification of the intermediate benzyl alcohol to form benzoic acid. This is achieved by treating the intermediate with a strong acid, such as hydrochloric acid (HCl) or sulfuric acid (H2SO4). The acidification process converts the alcohol group (-OH) into a carboxylic acid group (-COOH), resulting in the formation of benzoic acid.
**Conclusion:**
In summary, the conversion of methyl benzene to benzoic acid involves the oxidation of the methyl group to benzyl alcohol, followed by the oxidation of benzyl alcohol to benzoic acid, and finally the acidification of the intermediate to form benzoic acid. This multi-step process requires the use of strong oxidizing agents and acids to drive the desired chemical transformations.
**Introduction:**
The conversion of methyl benzene (toluene) to benzoic acid involves a series of chemical reactions that change the structure of the molecule, leading to the formation of benzoic acid. This process typically involves oxidation and subsequent acidification of the methyl group on the benzene ring.
**Step 1: Oxidation of Methyl Benzene**
The first step in the conversion of methyl benzene to benzoic acid is the oxidation of the methyl group to a carboxylic acid group. This can be achieved through a variety of oxidation methods, but one commonly used approach is the reaction with a strong oxidizing agent such as potassium permanganate (KMnO4) or chromic acid (H2CrO4). The oxidation of the methyl group results in the formation of a carboxylic acid group, giving rise to the intermediate molecule, benzyl alcohol.
**Step 2: Oxidation of Benzyl Alcohol**
The second step involves the further oxidation of benzyl alcohol to benzoic acid. This step is typically carried out by using a strong oxidizing agent such as potassium permanganate (KMnO4). The oxidation of benzyl alcohol leads to the formation of benzoic acid.
**Step 3: Acidification of the Intermediate**
The final step is the acidification of the intermediate benzyl alcohol to form benzoic acid. This is achieved by treating the intermediate with a strong acid, such as hydrochloric acid (HCl) or sulfuric acid (H2SO4). The acidification process converts the alcohol group (-OH) into a carboxylic acid group (-COOH), resulting in the formation of benzoic acid.
**Conclusion:**
In summary, the conversion of methyl benzene to benzoic acid involves the oxidation of the methyl group to benzyl alcohol, followed by the oxidation of benzyl alcohol to benzoic acid, and finally the acidification of the intermediate to form benzoic acid. This multi-step process requires the use of strong oxidizing agents and acids to drive the desired chemical transformations.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Benzoic acid from methyl benzene?
Question Description
Benzoic acid from methyl benzene? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Benzoic acid from methyl benzene? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Benzoic acid from methyl benzene?.
Benzoic acid from methyl benzene? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Benzoic acid from methyl benzene? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Benzoic acid from methyl benzene?.
Solutions for Benzoic acid from methyl benzene? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Benzoic acid from methyl benzene? defined & explained in the simplest way possible. Besides giving the explanation of
Benzoic acid from methyl benzene?, a detailed solution for Benzoic acid from methyl benzene? has been provided alongside types of Benzoic acid from methyl benzene? theory, EduRev gives you an
ample number of questions to practice Benzoic acid from methyl benzene? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.





















