Physics Exam > Physics Questions > Which is/are correct for fccstructure?a)packi...
Start Learning for Free
Which is/are correct for fcc structure?
- a)packing factor is 68%
- b)number of atoms in unit cell are 4
- c)number of atoms in unit cell are 2
- d)packing factor is 74%
Correct answer is option 'B,D'. Can you explain this answer?
Verified Answer
Which is/are correct for fccstructure?a)packing factor is 68%b)number ...
packing fraction for fcc is 74% and 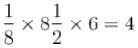 atoms / unit cell.
atoms / unit cell.
The correct answers are: number of atoms in unit cell are 4, packing factor is 74%
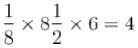 atoms / unit cell.
atoms / unit cell.The correct answers are: number of atoms in unit cell are 4, packing factor is 74%
Most Upvoted Answer
Which is/are correct for fccstructure?a)packing factor is 68%b)number ...
FCC Structure:
The FCC (Face-Centered Cubic) structure is a common crystal structure found in many metallic elements and alloys. It is characterized by a unit cell that has atoms at each of the eight corners and in the center of each face of the cube. The arrangement of atoms in the FCC structure is such that it maximizes the packing efficiency.
Packing Factor:
The packing factor is a measure of how efficiently the atoms are packed in a crystal structure. It is defined as the ratio of the total volume of atoms in the unit cell to the volume of the unit cell. For the FCC structure, the packing factor is given by the formula:
Packing factor = (Number of atoms in the unit cell * Volume of each atom) / Volume of the unit cell
Number of Atoms in the Unit Cell:
In the FCC structure, there are four atoms present in the unit cell. This can be understood by considering that there are atoms at each of the eight corners of the cube, and each atom is shared equally between eight neighboring unit cells. Therefore, each unit cell contributes 1/8th of an atom, resulting in a total of four atoms in the unit cell.
Packing Factor Calculation:
To calculate the packing factor for the FCC structure, we need to know the volume of each atom and the volume of the unit cell. The volume of each atom can be approximated by assuming it to be a sphere and using the formula for the volume of a sphere, which is (4/3) * π * r^3, where r is the radius of the atom.
The volume of the unit cell can be calculated by multiplying the length of one side of the cube by itself three times, since all sides of the cube are equal in the FCC structure.
Using these values, we can calculate the packing factor as follows:
Packing factor = (4 * (4/3) * π * r^3) / (a^3)
where r is the radius of the atom and a is the length of one side of the cube.
Correct Answer:
Based on the above explanation, the correct answers for the given options are:
b) The number of atoms in the unit cell is 4
d) The packing factor is 74%
The FCC (Face-Centered Cubic) structure is a common crystal structure found in many metallic elements and alloys. It is characterized by a unit cell that has atoms at each of the eight corners and in the center of each face of the cube. The arrangement of atoms in the FCC structure is such that it maximizes the packing efficiency.
Packing Factor:
The packing factor is a measure of how efficiently the atoms are packed in a crystal structure. It is defined as the ratio of the total volume of atoms in the unit cell to the volume of the unit cell. For the FCC structure, the packing factor is given by the formula:
Packing factor = (Number of atoms in the unit cell * Volume of each atom) / Volume of the unit cell
Number of Atoms in the Unit Cell:
In the FCC structure, there are four atoms present in the unit cell. This can be understood by considering that there are atoms at each of the eight corners of the cube, and each atom is shared equally between eight neighboring unit cells. Therefore, each unit cell contributes 1/8th of an atom, resulting in a total of four atoms in the unit cell.
Packing Factor Calculation:
To calculate the packing factor for the FCC structure, we need to know the volume of each atom and the volume of the unit cell. The volume of each atom can be approximated by assuming it to be a sphere and using the formula for the volume of a sphere, which is (4/3) * π * r^3, where r is the radius of the atom.
The volume of the unit cell can be calculated by multiplying the length of one side of the cube by itself three times, since all sides of the cube are equal in the FCC structure.
Using these values, we can calculate the packing factor as follows:
Packing factor = (4 * (4/3) * π * r^3) / (a^3)
where r is the radius of the atom and a is the length of one side of the cube.
Correct Answer:
Based on the above explanation, the correct answers for the given options are:
b) The number of atoms in the unit cell is 4
d) The packing factor is 74%

|
Explore Courses for Physics exam
|

|
Question Description
Which is/are correct for fccstructure?a)packing factor is 68%b)number of atoms in unit cell are 4 c)number of atoms in unit cell are 2 d)packing factor is 74%Correct answer is option 'B,D'. Can you explain this answer? for Physics 2025 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about Which is/are correct for fccstructure?a)packing factor is 68%b)number of atoms in unit cell are 4 c)number of atoms in unit cell are 2 d)packing factor is 74%Correct answer is option 'B,D'. Can you explain this answer? covers all topics & solutions for Physics 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which is/are correct for fccstructure?a)packing factor is 68%b)number of atoms in unit cell are 4 c)number of atoms in unit cell are 2 d)packing factor is 74%Correct answer is option 'B,D'. Can you explain this answer?.
Which is/are correct for fccstructure?a)packing factor is 68%b)number of atoms in unit cell are 4 c)number of atoms in unit cell are 2 d)packing factor is 74%Correct answer is option 'B,D'. Can you explain this answer? for Physics 2025 is part of Physics preparation. The Question and answers have been prepared according to the Physics exam syllabus. Information about Which is/are correct for fccstructure?a)packing factor is 68%b)number of atoms in unit cell are 4 c)number of atoms in unit cell are 2 d)packing factor is 74%Correct answer is option 'B,D'. Can you explain this answer? covers all topics & solutions for Physics 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which is/are correct for fccstructure?a)packing factor is 68%b)number of atoms in unit cell are 4 c)number of atoms in unit cell are 2 d)packing factor is 74%Correct answer is option 'B,D'. Can you explain this answer?.
Solutions for Which is/are correct for fccstructure?a)packing factor is 68%b)number of atoms in unit cell are 4 c)number of atoms in unit cell are 2 d)packing factor is 74%Correct answer is option 'B,D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Physics.
Download more important topics, notes, lectures and mock test series for Physics Exam by signing up for free.
Here you can find the meaning of Which is/are correct for fccstructure?a)packing factor is 68%b)number of atoms in unit cell are 4 c)number of atoms in unit cell are 2 d)packing factor is 74%Correct answer is option 'B,D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which is/are correct for fccstructure?a)packing factor is 68%b)number of atoms in unit cell are 4 c)number of atoms in unit cell are 2 d)packing factor is 74%Correct answer is option 'B,D'. Can you explain this answer?, a detailed solution for Which is/are correct for fccstructure?a)packing factor is 68%b)number of atoms in unit cell are 4 c)number of atoms in unit cell are 2 d)packing factor is 74%Correct answer is option 'B,D'. Can you explain this answer? has been provided alongside types of Which is/are correct for fccstructure?a)packing factor is 68%b)number of atoms in unit cell are 4 c)number of atoms in unit cell are 2 d)packing factor is 74%Correct answer is option 'B,D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which is/are correct for fccstructure?a)packing factor is 68%b)number of atoms in unit cell are 4 c)number of atoms in unit cell are 2 d)packing factor is 74%Correct answer is option 'B,D'. Can you explain this answer? tests, examples and also practice Physics tests.

|
Explore Courses for Physics exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























