Class 12 Exam > Class 12 Questions > Which one is not true about catalyst ?a)The c...
Start Learning for Free
Which one is not true about catalyst ?
- a)The catalyst is unchanged at the end of the reaction
- b)A small quantity of catalyst is often sufficient to bring about considerable amount of reaction
- c)The catalyst accelerates the reaction
- d)In a reversible recation the catalyst alters the equilibrium position
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
Which one is not true about catalyst ?a)The catalyst is unchanged at t...
The correct answer is Option D.
The catalyst is unchanged chemically at the end of a reaction. During the reaction between the chemical intermediates and the reactants, the catalyst is regenerated.
The catalyst accelerated the reaction. A catalyst is a substance which speeds up a reaction but is chemically unchanged at the end of the reaction.
In a reversible reaction, the catalyst does not alter the equilibrium position but increases the rate by decreasing activation energy and temperature.
A small amount of catalyst is often sufficient to bring about a large change in reaction. Catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are able to react more readily with each other or with another reactant to form the desired end product.
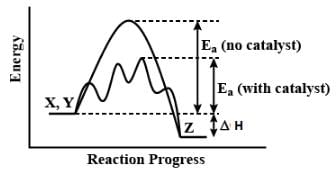
The catalyst accelerated the reaction. A catalyst is a substance which speeds up a reaction but is chemically unchanged at the end of the reaction.
In a reversible reaction, the catalyst does not alter the equilibrium position but increases the rate by decreasing activation energy and temperature.
A small amount of catalyst is often sufficient to bring about a large change in reaction. Catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are able to react more readily with each other or with another reactant to form the desired end product.

Most Upvoted Answer
Which one is not true about catalyst ?a)The catalyst is unchanged at t...
The correct answer is Option D.
The catalyst is unchanged chemically at the end of a reaction. During the reaction between the chemical intermediates and the reactants, the catalyst is regenerated.
The catalyst accelerated the reaction. A catalyst is a substance which speeds up a reaction but is chemically unchanged at the end of the reaction.
In a reversible reaction, the catalyst does not alter the equilibrium position but increases the rate by decreasing activation energy and temperature.
A small amount of catalyst is often sufficient to bring about a large change in reaction. Catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are able to react more readily with each other or with another reactant to form the desired end product.

The catalyst accelerated the reaction. A catalyst is a substance which speeds up a reaction but is chemically unchanged at the end of the reaction.
In a reversible reaction, the catalyst does not alter the equilibrium position but increases the rate by decreasing activation energy and temperature.
A small amount of catalyst is often sufficient to bring about a large change in reaction. Catalytic action is a chemical reaction between the catalyst and a reactant, forming chemical intermediates that are able to react more readily with each other or with another reactant to form the desired end product.

Free Test
FREE
| Start Free Test |
Community Answer
Which one is not true about catalyst ?a)The catalyst is unchanged at t...
In a reversible reaction the catalyst wouldn't alter equilibrium position, it will only increase rate of reaction because of which equilibrium position will be attained soon... So option (d) is correct...

|
Explore Courses for Class 12 exam
|

|
Question Description
Which one is not true about catalyst ?a)The catalyst is unchanged at the end of the reactionb)A small quantity of catalyst is often sufficient to bring about considerable amount of reactionc)The catalyst accelerates the reactiond)In a reversible recation the catalyst alters the equilibrium positionCorrect answer is option 'D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Which one is not true about catalyst ?a)The catalyst is unchanged at the end of the reactionb)A small quantity of catalyst is often sufficient to bring about considerable amount of reactionc)The catalyst accelerates the reactiond)In a reversible recation the catalyst alters the equilibrium positionCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which one is not true about catalyst ?a)The catalyst is unchanged at the end of the reactionb)A small quantity of catalyst is often sufficient to bring about considerable amount of reactionc)The catalyst accelerates the reactiond)In a reversible recation the catalyst alters the equilibrium positionCorrect answer is option 'D'. Can you explain this answer?.
Which one is not true about catalyst ?a)The catalyst is unchanged at the end of the reactionb)A small quantity of catalyst is often sufficient to bring about considerable amount of reactionc)The catalyst accelerates the reactiond)In a reversible recation the catalyst alters the equilibrium positionCorrect answer is option 'D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Which one is not true about catalyst ?a)The catalyst is unchanged at the end of the reactionb)A small quantity of catalyst is often sufficient to bring about considerable amount of reactionc)The catalyst accelerates the reactiond)In a reversible recation the catalyst alters the equilibrium positionCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which one is not true about catalyst ?a)The catalyst is unchanged at the end of the reactionb)A small quantity of catalyst is often sufficient to bring about considerable amount of reactionc)The catalyst accelerates the reactiond)In a reversible recation the catalyst alters the equilibrium positionCorrect answer is option 'D'. Can you explain this answer?.
Solutions for Which one is not true about catalyst ?a)The catalyst is unchanged at the end of the reactionb)A small quantity of catalyst is often sufficient to bring about considerable amount of reactionc)The catalyst accelerates the reactiond)In a reversible recation the catalyst alters the equilibrium positionCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Which one is not true about catalyst ?a)The catalyst is unchanged at the end of the reactionb)A small quantity of catalyst is often sufficient to bring about considerable amount of reactionc)The catalyst accelerates the reactiond)In a reversible recation the catalyst alters the equilibrium positionCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which one is not true about catalyst ?a)The catalyst is unchanged at the end of the reactionb)A small quantity of catalyst is often sufficient to bring about considerable amount of reactionc)The catalyst accelerates the reactiond)In a reversible recation the catalyst alters the equilibrium positionCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for Which one is not true about catalyst ?a)The catalyst is unchanged at the end of the reactionb)A small quantity of catalyst is often sufficient to bring about considerable amount of reactionc)The catalyst accelerates the reactiond)In a reversible recation the catalyst alters the equilibrium positionCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of Which one is not true about catalyst ?a)The catalyst is unchanged at the end of the reactionb)A small quantity of catalyst is often sufficient to bring about considerable amount of reactionc)The catalyst accelerates the reactiond)In a reversible recation the catalyst alters the equilibrium positionCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which one is not true about catalyst ?a)The catalyst is unchanged at the end of the reactionb)A small quantity of catalyst is often sufficient to bring about considerable amount of reactionc)The catalyst accelerates the reactiond)In a reversible recation the catalyst alters the equilibrium positionCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























