Class 12 Exam > Class 12 Questions > What Steps involved in preparation of human i...
Start Learning for Free
What Steps involved in preparation of human insulin ? Order wise please explain answer?
Most Upvoted Answer
What Steps involved in preparation of human insulin ? Order wise pleas...
Community Answer
What Steps involved in preparation of human insulin ? Order wise pleas...
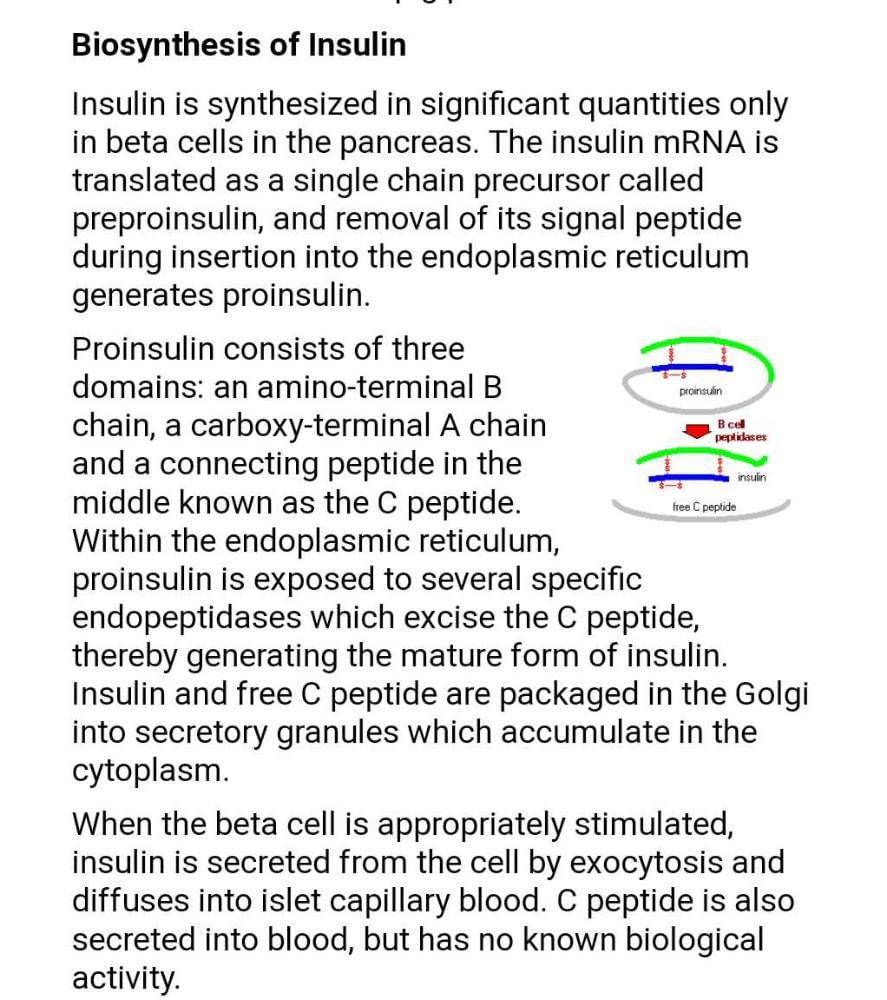
Preparation of Human Insulin
1. Isolation of Insulin mRNA:
The first step in the preparation of human insulin involves the isolation of insulin mRNA from the pancreas of a human donor. The pancreas is homogenized and the total RNA is extracted. The mRNA is then purified using techniques like gel electrophoresis or chromatography.
2. Synthesis of Complementary DNA (cDNA):
The isolated mRNA serves as a template for the synthesis of complementary DNA (cDNA) using the enzyme reverse transcriptase. This enzyme synthesizes a strand of DNA complementary to the mRNA sequence.
3. Amplification of cDNA:
The cDNA is then amplified using a technique called polymerase chain reaction (PCR). This process involves multiple cycles of DNA denaturation, primer annealing, and DNA synthesis. As a result, a large amount of cDNA is generated.
4. Cloning of cDNA into Plasmid Vector:
The amplified cDNA is ligated into a plasmid vector, which serves as a carrier for the cDNA. This recombinant DNA molecule is then introduced into a bacterial host, such as Escherichia coli, through a process called transformation.
5. Expression of Recombinant DNA:
The transformed bacteria are cultured under specific conditions that promote the expression of the recombinant DNA. The cDNA in the plasmid vector is transcribed into mRNA, which is then translated by the host's cellular machinery to produce human insulin.
6. Extraction and Purification of Insulin:
The bacterial cells are harvested and lysed to release the insulin produced. The cell debris is removed by centrifugation, and the insulin-containing supernatant is collected. The extracted insulin is then purified using techniques like chromatography, filtration, and precipitation.
7. Formulation and Packaging:
The purified insulin is formulated into a suitable pharmaceutical product, such as a solution or suspension. It may be mixed with stabilizers and preservatives to improve its shelf life. The final product is packaged in vials or cartridges for storage and distribution.
8. Quality Control:
The prepared human insulin undergoes rigorous quality control tests to ensure its safety, potency, and purity. These tests include identity verification, potency assay, sterility testing, and endotoxin testing. Only insulin batches that meet the required standards are released for use.
9. Distribution and Storage:
The final step involves the distribution and storage of the prepared human insulin. It is stored under controlled conditions to maintain its stability and efficacy until it reaches the end-users.
Overall, the preparation of human insulin involves a series of steps including isolation of insulin mRNA, synthesis and amplification of cDNA, cloning into a plasmid vector, expression in bacteria, extraction and purification, formulation and packaging, quality control, and distribution. These steps ensure the production of high-quality human insulin for the treatment of diabetes.
1. Isolation of Insulin mRNA:
The first step in the preparation of human insulin involves the isolation of insulin mRNA from the pancreas of a human donor. The pancreas is homogenized and the total RNA is extracted. The mRNA is then purified using techniques like gel electrophoresis or chromatography.
2. Synthesis of Complementary DNA (cDNA):
The isolated mRNA serves as a template for the synthesis of complementary DNA (cDNA) using the enzyme reverse transcriptase. This enzyme synthesizes a strand of DNA complementary to the mRNA sequence.
3. Amplification of cDNA:
The cDNA is then amplified using a technique called polymerase chain reaction (PCR). This process involves multiple cycles of DNA denaturation, primer annealing, and DNA synthesis. As a result, a large amount of cDNA is generated.
4. Cloning of cDNA into Plasmid Vector:
The amplified cDNA is ligated into a plasmid vector, which serves as a carrier for the cDNA. This recombinant DNA molecule is then introduced into a bacterial host, such as Escherichia coli, through a process called transformation.
5. Expression of Recombinant DNA:
The transformed bacteria are cultured under specific conditions that promote the expression of the recombinant DNA. The cDNA in the plasmid vector is transcribed into mRNA, which is then translated by the host's cellular machinery to produce human insulin.
6. Extraction and Purification of Insulin:
The bacterial cells are harvested and lysed to release the insulin produced. The cell debris is removed by centrifugation, and the insulin-containing supernatant is collected. The extracted insulin is then purified using techniques like chromatography, filtration, and precipitation.
7. Formulation and Packaging:
The purified insulin is formulated into a suitable pharmaceutical product, such as a solution or suspension. It may be mixed with stabilizers and preservatives to improve its shelf life. The final product is packaged in vials or cartridges for storage and distribution.
8. Quality Control:
The prepared human insulin undergoes rigorous quality control tests to ensure its safety, potency, and purity. These tests include identity verification, potency assay, sterility testing, and endotoxin testing. Only insulin batches that meet the required standards are released for use.
9. Distribution and Storage:
The final step involves the distribution and storage of the prepared human insulin. It is stored under controlled conditions to maintain its stability and efficacy until it reaches the end-users.
Overall, the preparation of human insulin involves a series of steps including isolation of insulin mRNA, synthesis and amplification of cDNA, cloning into a plasmid vector, expression in bacteria, extraction and purification, formulation and packaging, quality control, and distribution. These steps ensure the production of high-quality human insulin for the treatment of diabetes.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
What Steps involved in preparation of human insulin ? Order wise please explain answer?
Question Description
What Steps involved in preparation of human insulin ? Order wise please explain answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about What Steps involved in preparation of human insulin ? Order wise please explain answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What Steps involved in preparation of human insulin ? Order wise please explain answer?.
What Steps involved in preparation of human insulin ? Order wise please explain answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about What Steps involved in preparation of human insulin ? Order wise please explain answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What Steps involved in preparation of human insulin ? Order wise please explain answer?.
Solutions for What Steps involved in preparation of human insulin ? Order wise please explain answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of What Steps involved in preparation of human insulin ? Order wise please explain answer? defined & explained in the simplest way possible. Besides giving the explanation of
What Steps involved in preparation of human insulin ? Order wise please explain answer?, a detailed solution for What Steps involved in preparation of human insulin ? Order wise please explain answer? has been provided alongside types of What Steps involved in preparation of human insulin ? Order wise please explain answer? theory, EduRev gives you an
ample number of questions to practice What Steps involved in preparation of human insulin ? Order wise please explain answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















