Class 12 Exam > Class 12 Questions > Differentiate between multi-molecular and mac...
Start Learning for Free
Differentiate between multi-molecular and macromolecular colloids? Give one example of each. How are these two types of colloids different from associated colloids?
Most Upvoted Answer
Differentiate between multi-molecular and macromolecular colloids? Giv...
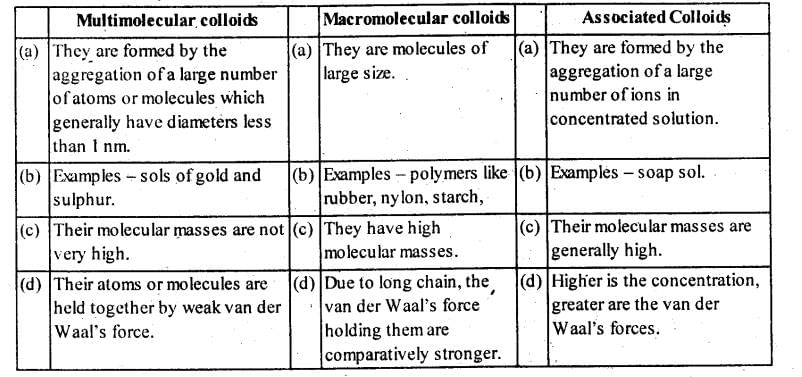
Community Answer
Differentiate between multi-molecular and macromolecular colloids? Giv...
Multi-molecular Colloids
- Multi-molecular colloids are formed by the aggregation of molecules to form particles in the colloidal range.
- These colloids are relatively smaller in size compared to macromolecular colloids.
- Example: Gold sol, where gold particles are dispersed in a solvent.
Macromolecular Colloids
- Macromolecular colloids are formed by the aggregation of large molecules or polymers to form colloidal particles.
- These colloids are larger in size compared to multi-molecular colloids.
- Example: Starch solution, where starch molecules form colloidal particles in water.
Difference from Associated Colloids
- Multi-molecular and macromolecular colloids differ from associated colloids in terms of the nature of the particles involved.
- Multi-molecular and macromolecular colloids are formed by the physical aggregation of individual molecules or polymers, whereas associated colloids are formed by the association of molecules through weak intermolecular forces like hydrogen bonding.
- Multi-molecular and macromolecular colloids have stable particles that do not dissociate easily, while associated colloids can dissociate into individual molecules under certain conditions.
- The size of particles in multi-molecular and macromolecular colloids is relatively larger compared to associated colloids, which have smaller particle sizes.
- Examples of associated colloids include micelles formed by the aggregation of surfactant molecules in water.
In conclusion, multi-molecular and macromolecular colloids are distinguished by the size and nature of particles involved, while associated colloids are characterized by the weak intermolecular forces that hold the particles together. Each type of colloid has unique properties and applications in various fields.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Question Description
Differentiate between multi-molecular and macromolecular colloids? Give one example of each. How are these two types of colloids different from associated colloids? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Differentiate between multi-molecular and macromolecular colloids? Give one example of each. How are these two types of colloids different from associated colloids? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Differentiate between multi-molecular and macromolecular colloids? Give one example of each. How are these two types of colloids different from associated colloids?.
Differentiate between multi-molecular and macromolecular colloids? Give one example of each. How are these two types of colloids different from associated colloids? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Differentiate between multi-molecular and macromolecular colloids? Give one example of each. How are these two types of colloids different from associated colloids? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Differentiate between multi-molecular and macromolecular colloids? Give one example of each. How are these two types of colloids different from associated colloids?.
Solutions for Differentiate between multi-molecular and macromolecular colloids? Give one example of each. How are these two types of colloids different from associated colloids? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Differentiate between multi-molecular and macromolecular colloids? Give one example of each. How are these two types of colloids different from associated colloids? defined & explained in the simplest way possible. Besides giving the explanation of
Differentiate between multi-molecular and macromolecular colloids? Give one example of each. How are these two types of colloids different from associated colloids?, a detailed solution for Differentiate between multi-molecular and macromolecular colloids? Give one example of each. How are these two types of colloids different from associated colloids? has been provided alongside types of Differentiate between multi-molecular and macromolecular colloids? Give one example of each. How are these two types of colloids different from associated colloids? theory, EduRev gives you an
ample number of questions to practice Differentiate between multi-molecular and macromolecular colloids? Give one example of each. How are these two types of colloids different from associated colloids? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.




















