JEE Exam > JEE Questions > Statement-1: An electron and a proton are acc...
Start Learning for Free
Statement-1: An electron and a proton are accelerated through the same potential difference. The deBroglie wavelength associated with the electron is longer.
Statement-2: De-Broglie wavelength associated with a moving particle is l = 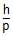 where, p is the linear momentum and both have same K.E.
where, p is the linear momentum and both have same K.E.
- a)Statement-1 is true, statement-2 is true and statement-2 is correct explanation for statement-1.
- b)Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1.
- c)Statement-1 is true, statement-2 is false.
- d)Statement-1 is false, statement-2 is true.
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Statement-1: An electron and a proton are accelerated through the same...
de Broglie Wavelength for Electrons
In the case of electrons going in circles around the nuclei in atoms, the de Broglie waves exist as a closed-loop, such that they can exist only as standing waves, and fit evenly around the loop. Because of this requirement, the electrons in atoms circle the nucleus in particular configurations, or states, which are called stationary orbits.
de Broglie Wavelength Formula and Derivation
de Broglie reasoned that matter also can show wave-particle duality, just like light, since light can behave both as a wave (it can be diffracted and it has a wavelength) and as a particle (it contains packets of energy hν). And also reasoned that matter would follow the same equation for wavelength as light namely,
λ = h / p
Where p is the linear momentum, as shown by Einstein.
Derivation
de Broglie derived the above relationship as follows:
1) E = hν for a photon and λν = c for an electromagnetic wave.
2) E = mc2, means λ = h/mc, which is equivalent to λ = h/p.
Note: m is the relativistic mass, and not the rest mass; since the rest mass of a photon is zero.
Now, if a particle is moving with a velocity v, the momentum p = mv and hence λ = h / mv
Therefore, the de Broglie wavelength formula is expressed as;
λ = h / mv
In the case of electrons going in circles around the nuclei in atoms, the de Broglie waves exist as a closed-loop, such that they can exist only as standing waves, and fit evenly around the loop. Because of this requirement, the electrons in atoms circle the nucleus in particular configurations, or states, which are called stationary orbits.
de Broglie Wavelength Formula and Derivation
de Broglie reasoned that matter also can show wave-particle duality, just like light, since light can behave both as a wave (it can be diffracted and it has a wavelength) and as a particle (it contains packets of energy hν). And also reasoned that matter would follow the same equation for wavelength as light namely,
λ = h / p
Where p is the linear momentum, as shown by Einstein.
Derivation
de Broglie derived the above relationship as follows:
1) E = hν for a photon and λν = c for an electromagnetic wave.
2) E = mc2, means λ = h/mc, which is equivalent to λ = h/p.
Note: m is the relativistic mass, and not the rest mass; since the rest mass of a photon is zero.
Now, if a particle is moving with a velocity v, the momentum p = mv and hence λ = h / mv
Therefore, the de Broglie wavelength formula is expressed as;
λ = h / mv

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Statement-1: An electron and a proton are accelerated through the same potential difference. The deBroglie wavelength associated with the electron is longer.Statement-2: De-Broglie wavelength associated with a moving particle is l =where, p is the linear momentum and both have same K.E.a)Statement-1 is true, statement-2 is true and statement-2 is correct explanation for statement-1.b)Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1.c)Statement-1 is true, statement-2 is false.d)Statement-1 is false, statement-2 is true.Correct answer is option 'A'. Can you explain this answer?
Question Description
Statement-1: An electron and a proton are accelerated through the same potential difference. The deBroglie wavelength associated with the electron is longer.Statement-2: De-Broglie wavelength associated with a moving particle is l =where, p is the linear momentum and both have same K.E.a)Statement-1 is true, statement-2 is true and statement-2 is correct explanation for statement-1.b)Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1.c)Statement-1 is true, statement-2 is false.d)Statement-1 is false, statement-2 is true.Correct answer is option 'A'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Statement-1: An electron and a proton are accelerated through the same potential difference. The deBroglie wavelength associated with the electron is longer.Statement-2: De-Broglie wavelength associated with a moving particle is l =where, p is the linear momentum and both have same K.E.a)Statement-1 is true, statement-2 is true and statement-2 is correct explanation for statement-1.b)Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1.c)Statement-1 is true, statement-2 is false.d)Statement-1 is false, statement-2 is true.Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Statement-1: An electron and a proton are accelerated through the same potential difference. The deBroglie wavelength associated with the electron is longer.Statement-2: De-Broglie wavelength associated with a moving particle is l =where, p is the linear momentum and both have same K.E.a)Statement-1 is true, statement-2 is true and statement-2 is correct explanation for statement-1.b)Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1.c)Statement-1 is true, statement-2 is false.d)Statement-1 is false, statement-2 is true.Correct answer is option 'A'. Can you explain this answer?.
Statement-1: An electron and a proton are accelerated through the same potential difference. The deBroglie wavelength associated with the electron is longer.Statement-2: De-Broglie wavelength associated with a moving particle is l =where, p is the linear momentum and both have same K.E.a)Statement-1 is true, statement-2 is true and statement-2 is correct explanation for statement-1.b)Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1.c)Statement-1 is true, statement-2 is false.d)Statement-1 is false, statement-2 is true.Correct answer is option 'A'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Statement-1: An electron and a proton are accelerated through the same potential difference. The deBroglie wavelength associated with the electron is longer.Statement-2: De-Broglie wavelength associated with a moving particle is l =where, p is the linear momentum and both have same K.E.a)Statement-1 is true, statement-2 is true and statement-2 is correct explanation for statement-1.b)Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1.c)Statement-1 is true, statement-2 is false.d)Statement-1 is false, statement-2 is true.Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Statement-1: An electron and a proton are accelerated through the same potential difference. The deBroglie wavelength associated with the electron is longer.Statement-2: De-Broglie wavelength associated with a moving particle is l =where, p is the linear momentum and both have same K.E.a)Statement-1 is true, statement-2 is true and statement-2 is correct explanation for statement-1.b)Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1.c)Statement-1 is true, statement-2 is false.d)Statement-1 is false, statement-2 is true.Correct answer is option 'A'. Can you explain this answer?.
Solutions for Statement-1: An electron and a proton are accelerated through the same potential difference. The deBroglie wavelength associated with the electron is longer.Statement-2: De-Broglie wavelength associated with a moving particle is l =where, p is the linear momentum and both have same K.E.a)Statement-1 is true, statement-2 is true and statement-2 is correct explanation for statement-1.b)Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1.c)Statement-1 is true, statement-2 is false.d)Statement-1 is false, statement-2 is true.Correct answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Statement-1: An electron and a proton are accelerated through the same potential difference. The deBroglie wavelength associated with the electron is longer.Statement-2: De-Broglie wavelength associated with a moving particle is l =where, p is the linear momentum and both have same K.E.a)Statement-1 is true, statement-2 is true and statement-2 is correct explanation for statement-1.b)Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1.c)Statement-1 is true, statement-2 is false.d)Statement-1 is false, statement-2 is true.Correct answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Statement-1: An electron and a proton are accelerated through the same potential difference. The deBroglie wavelength associated with the electron is longer.Statement-2: De-Broglie wavelength associated with a moving particle is l =where, p is the linear momentum and both have same K.E.a)Statement-1 is true, statement-2 is true and statement-2 is correct explanation for statement-1.b)Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1.c)Statement-1 is true, statement-2 is false.d)Statement-1 is false, statement-2 is true.Correct answer is option 'A'. Can you explain this answer?, a detailed solution for Statement-1: An electron and a proton are accelerated through the same potential difference. The deBroglie wavelength associated with the electron is longer.Statement-2: De-Broglie wavelength associated with a moving particle is l =where, p is the linear momentum and both have same K.E.a)Statement-1 is true, statement-2 is true and statement-2 is correct explanation for statement-1.b)Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1.c)Statement-1 is true, statement-2 is false.d)Statement-1 is false, statement-2 is true.Correct answer is option 'A'. Can you explain this answer? has been provided alongside types of Statement-1: An electron and a proton are accelerated through the same potential difference. The deBroglie wavelength associated with the electron is longer.Statement-2: De-Broglie wavelength associated with a moving particle is l =where, p is the linear momentum and both have same K.E.a)Statement-1 is true, statement-2 is true and statement-2 is correct explanation for statement-1.b)Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1.c)Statement-1 is true, statement-2 is false.d)Statement-1 is false, statement-2 is true.Correct answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Statement-1: An electron and a proton are accelerated through the same potential difference. The deBroglie wavelength associated with the electron is longer.Statement-2: De-Broglie wavelength associated with a moving particle is l =where, p is the linear momentum and both have same K.E.a)Statement-1 is true, statement-2 is true and statement-2 is correct explanation for statement-1.b)Statement-1 is true, statement-2 is true and statement-2 is NOT the correct explanation for statement-1.c)Statement-1 is true, statement-2 is false.d)Statement-1 is false, statement-2 is true.Correct answer is option 'A'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























