JEE Exam > JEE Questions > Titrations are one of the methods we can use ...
Start Learning for Free
Titrations are one of the methods we can use to discover the precise concentrations of solution. A typical titration involves adding a solution from a burette to another solution in a flask. The endpoint of the titration is found by watching a colour change taking place. However, a problem arises when a suitable indicator cannot be found, or when the colour changes involved are unclear. In cases redox potential may sometimes come to the rescue.
A particularly well known example (Fig.1) is a method of discovering the concentration of iron in a solution by titrating them with a solution of cerium (IV). The redox potential that are
of interest here are E°Fe3+/Fe2+ = + 0.77 V and E°Ce4+/Ce3+ = + 1.61 V. These tell us that cercium (IV) ions are the oxidizing agents, and iron (II) ions are the reducing agent. They should react according to the equation
Fe2+ (aq) + Ce4+ (aq) → Fe3+ (aq) + Ce3+ (aq)
Now imagine that we know the concentration of the cerium (IV) ions solution in the burette. We want to measure the concentration of the iron (II) solution. If we add just one drop of the cerium (IV) solution from the bruette, some of the iron (II) ions will be oxidised. As a consequence the beaker would now contain a large number of unreacted ions, but also some iron (III) ions as well. All of the cerium (III). The solution in the beaker now represents an iron(III)/iron(II) half cell, although not at standard conditions. Thus the e.m.f. of the cell will be near, but not equal, to E°Fe3+/Fe2+.
to ad cerium (IV) solution, the number of iron (II) ions is gradually reduced and eventually only a very few are left (Table).At this stage the next few drops of cerium (IV) solution convert all the remaining iron (II) ions into iron (III), and some of the cerium (IV) ions are left unreacted. Once this happens we no longer ions and a smaller number of cerium (IV) ions. The solution in the beaker now behaves as a cerium (IV)/cerium (III) half-cell (although not a standard one).
Just before all the iron (II) ions are converted into iron (III) we have a cell with an e.m.f.of around + 0.77 V. After all the iron (II) ions are oxidised, we have a cell with an e.m.f. of about + 1.61 V. This rapid rise in e.m.f. occurs with the addition of hust one drop of cerium (IV) solution. You should be able to understand why a graph of cell e.m.f. against volume of
cerium (IV) solution added looks like that of Fig. 2. The end point of the titration can be read from the graph and the concentration of the iron (II) solution calculated in the usual way
Q.
Imagine you were given a solution of potassium dichromate (VI) in a beaker, and a solution of iron (II) sulphate in a burette. You do not know the concentration of dichromate (VI) ions, but the concentration of the iron (II) solution is known. Your task is to carry out a redox titration using the two solutions in order to determine the concentration of dichromate(VI) ions. Sketch a graph how the e.m.f. changes in the course of above titration. E° Cr2O2-7/Cr3+ = 1.33 V, E°Fe3+/Fe2+ = 0.77 V.
- a)
- b)
- c)
- d)
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Titrations are one of the methods we can use to discover the precise c...
After one drop iron (II) solution is added the beaker will contain a mixture of Cr2O72-, Cr3+ and Fe3+ ions. The e.m.f. will be near to 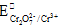
= + 0.77. The reaction is:
6Fe2+ (aq) + Cr2O72- (aq) + 14H+ (aq) → 6Fe3+ (aq) + 2Cr3+ (aq) + 7H2O(I)
The apparatus would be like that in figure. The graph is shown is figure.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Titrations are one of the methods we can use to discover the precise concentrations of solution. A typical titration involves adding a solution from a burette to another solution in a flask. The endpoint of the titration is found by watching a colour change taking place. However, a problem arises when a suitable indicator cannot be found, or when the colour changes involved are unclear. In cases redox potential may sometimes come to the rescue.A particularly well known example (Fig.1) is a method of discovering the concentration of iron in a solution by titrating them with a solution of cerium (IV). The redox potential that areof interest here are EFe3+/Fe2+ = + 0.77 V and ECe4+/Ce3+ = + 1.61 V. These tell us that cercium (IV) ions are the oxidizing agents, and iron (II) ions are the reducing agent. They should react according to the equationFe2+ (aq) + Ce4+ (aq) Fe3+ (aq) + Ce3+ (aq)Now imagine that we know the concentration of the cerium (IV) ions solution in the burette. We want to measure the concentration of the iron (II) solution. If we add just one drop of the cerium (IV) solution from the bruette, some of the iron (II) ions will be oxidised. As a consequence the beaker would now contain a large number of unreacted ions, but also some iron (III) ions as well. All of the cerium (III). The solution in the beaker now represents an iron(III)/iron(II) half cell, although not at standard conditions. Thus the e.m.f. of the cell will be near, but not equal, to EFe3+/Fe2+.to ad cerium (IV) solution, the number of iron (II) ions is gradually reduced and eventually only a very few are left (Table).At this stage the next few drops of cerium (IV) solution convert all the remaining iron (II) ions into iron (III), and some of the cerium (IV) ions are left unreacted. Once this happens we no longer ions and a smaller number of cerium (IV) ions. The solution in the beaker now behaves as a cerium (IV)/cerium (III) half-cell (although not a standard one).Just before all the iron (II) ions are converted into iron (III) we have a cell with an e.m.f.of around + 0.77 V. After all the iron (II) ions are oxidised, we have a cell with an e.m.f. of about + 1.61 V. This rapid rise in e.m.f. occurs with the addition of hust one drop of cerium (IV) solution. You should be able to understand why a graph of cell e.m.f. against volume ofcerium (IV) solution added looks like that of Fig. 2. The end point of the titration can be read from the graph and the concentration of the iron (II) solution calculated in the usual wayQ.Imagine you were given a solution of potassium dichromate (VI) in a beaker, and a solution of iron (II) sulphate in a burette. You do not know the concentration of dichromate (VI) ions, but the concentration of the iron (II) solution is known. Your task is to carry out a redox titration using the two solutions in order to determine the concentration of dichromate(VI) ions. Sketch a graph how the e.m.f. changes in the course of above titration. E Cr2O2-7/Cr3+ = 1.33 V, EFe3+/Fe2+ = 0.77 V.a)b)c)d)Correct answer is option 'B'. Can you explain this answer?
Question Description
Titrations are one of the methods we can use to discover the precise concentrations of solution. A typical titration involves adding a solution from a burette to another solution in a flask. The endpoint of the titration is found by watching a colour change taking place. However, a problem arises when a suitable indicator cannot be found, or when the colour changes involved are unclear. In cases redox potential may sometimes come to the rescue.A particularly well known example (Fig.1) is a method of discovering the concentration of iron in a solution by titrating them with a solution of cerium (IV). The redox potential that areof interest here are EFe3+/Fe2+ = + 0.77 V and ECe4+/Ce3+ = + 1.61 V. These tell us that cercium (IV) ions are the oxidizing agents, and iron (II) ions are the reducing agent. They should react according to the equationFe2+ (aq) + Ce4+ (aq) Fe3+ (aq) + Ce3+ (aq)Now imagine that we know the concentration of the cerium (IV) ions solution in the burette. We want to measure the concentration of the iron (II) solution. If we add just one drop of the cerium (IV) solution from the bruette, some of the iron (II) ions will be oxidised. As a consequence the beaker would now contain a large number of unreacted ions, but also some iron (III) ions as well. All of the cerium (III). The solution in the beaker now represents an iron(III)/iron(II) half cell, although not at standard conditions. Thus the e.m.f. of the cell will be near, but not equal, to EFe3+/Fe2+.to ad cerium (IV) solution, the number of iron (II) ions is gradually reduced and eventually only a very few are left (Table).At this stage the next few drops of cerium (IV) solution convert all the remaining iron (II) ions into iron (III), and some of the cerium (IV) ions are left unreacted. Once this happens we no longer ions and a smaller number of cerium (IV) ions. The solution in the beaker now behaves as a cerium (IV)/cerium (III) half-cell (although not a standard one).Just before all the iron (II) ions are converted into iron (III) we have a cell with an e.m.f.of around + 0.77 V. After all the iron (II) ions are oxidised, we have a cell with an e.m.f. of about + 1.61 V. This rapid rise in e.m.f. occurs with the addition of hust one drop of cerium (IV) solution. You should be able to understand why a graph of cell e.m.f. against volume ofcerium (IV) solution added looks like that of Fig. 2. The end point of the titration can be read from the graph and the concentration of the iron (II) solution calculated in the usual wayQ.Imagine you were given a solution of potassium dichromate (VI) in a beaker, and a solution of iron (II) sulphate in a burette. You do not know the concentration of dichromate (VI) ions, but the concentration of the iron (II) solution is known. Your task is to carry out a redox titration using the two solutions in order to determine the concentration of dichromate(VI) ions. Sketch a graph how the e.m.f. changes in the course of above titration. E Cr2O2-7/Cr3+ = 1.33 V, EFe3+/Fe2+ = 0.77 V.a)b)c)d)Correct answer is option 'B'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Titrations are one of the methods we can use to discover the precise concentrations of solution. A typical titration involves adding a solution from a burette to another solution in a flask. The endpoint of the titration is found by watching a colour change taking place. However, a problem arises when a suitable indicator cannot be found, or when the colour changes involved are unclear. In cases redox potential may sometimes come to the rescue.A particularly well known example (Fig.1) is a method of discovering the concentration of iron in a solution by titrating them with a solution of cerium (IV). The redox potential that areof interest here are EFe3+/Fe2+ = + 0.77 V and ECe4+/Ce3+ = + 1.61 V. These tell us that cercium (IV) ions are the oxidizing agents, and iron (II) ions are the reducing agent. They should react according to the equationFe2+ (aq) + Ce4+ (aq) Fe3+ (aq) + Ce3+ (aq)Now imagine that we know the concentration of the cerium (IV) ions solution in the burette. We want to measure the concentration of the iron (II) solution. If we add just one drop of the cerium (IV) solution from the bruette, some of the iron (II) ions will be oxidised. As a consequence the beaker would now contain a large number of unreacted ions, but also some iron (III) ions as well. All of the cerium (III). The solution in the beaker now represents an iron(III)/iron(II) half cell, although not at standard conditions. Thus the e.m.f. of the cell will be near, but not equal, to EFe3+/Fe2+.to ad cerium (IV) solution, the number of iron (II) ions is gradually reduced and eventually only a very few are left (Table).At this stage the next few drops of cerium (IV) solution convert all the remaining iron (II) ions into iron (III), and some of the cerium (IV) ions are left unreacted. Once this happens we no longer ions and a smaller number of cerium (IV) ions. The solution in the beaker now behaves as a cerium (IV)/cerium (III) half-cell (although not a standard one).Just before all the iron (II) ions are converted into iron (III) we have a cell with an e.m.f.of around + 0.77 V. After all the iron (II) ions are oxidised, we have a cell with an e.m.f. of about + 1.61 V. This rapid rise in e.m.f. occurs with the addition of hust one drop of cerium (IV) solution. You should be able to understand why a graph of cell e.m.f. against volume ofcerium (IV) solution added looks like that of Fig. 2. The end point of the titration can be read from the graph and the concentration of the iron (II) solution calculated in the usual wayQ.Imagine you were given a solution of potassium dichromate (VI) in a beaker, and a solution of iron (II) sulphate in a burette. You do not know the concentration of dichromate (VI) ions, but the concentration of the iron (II) solution is known. Your task is to carry out a redox titration using the two solutions in order to determine the concentration of dichromate(VI) ions. Sketch a graph how the e.m.f. changes in the course of above titration. E Cr2O2-7/Cr3+ = 1.33 V, EFe3+/Fe2+ = 0.77 V.a)b)c)d)Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Titrations are one of the methods we can use to discover the precise concentrations of solution. A typical titration involves adding a solution from a burette to another solution in a flask. The endpoint of the titration is found by watching a colour change taking place. However, a problem arises when a suitable indicator cannot be found, or when the colour changes involved are unclear. In cases redox potential may sometimes come to the rescue.A particularly well known example (Fig.1) is a method of discovering the concentration of iron in a solution by titrating them with a solution of cerium (IV). The redox potential that areof interest here are EFe3+/Fe2+ = + 0.77 V and ECe4+/Ce3+ = + 1.61 V. These tell us that cercium (IV) ions are the oxidizing agents, and iron (II) ions are the reducing agent. They should react according to the equationFe2+ (aq) + Ce4+ (aq) Fe3+ (aq) + Ce3+ (aq)Now imagine that we know the concentration of the cerium (IV) ions solution in the burette. We want to measure the concentration of the iron (II) solution. If we add just one drop of the cerium (IV) solution from the bruette, some of the iron (II) ions will be oxidised. As a consequence the beaker would now contain a large number of unreacted ions, but also some iron (III) ions as well. All of the cerium (III). The solution in the beaker now represents an iron(III)/iron(II) half cell, although not at standard conditions. Thus the e.m.f. of the cell will be near, but not equal, to EFe3+/Fe2+.to ad cerium (IV) solution, the number of iron (II) ions is gradually reduced and eventually only a very few are left (Table).At this stage the next few drops of cerium (IV) solution convert all the remaining iron (II) ions into iron (III), and some of the cerium (IV) ions are left unreacted. Once this happens we no longer ions and a smaller number of cerium (IV) ions. The solution in the beaker now behaves as a cerium (IV)/cerium (III) half-cell (although not a standard one).Just before all the iron (II) ions are converted into iron (III) we have a cell with an e.m.f.of around + 0.77 V. After all the iron (II) ions are oxidised, we have a cell with an e.m.f. of about + 1.61 V. This rapid rise in e.m.f. occurs with the addition of hust one drop of cerium (IV) solution. You should be able to understand why a graph of cell e.m.f. against volume ofcerium (IV) solution added looks like that of Fig. 2. The end point of the titration can be read from the graph and the concentration of the iron (II) solution calculated in the usual wayQ.Imagine you were given a solution of potassium dichromate (VI) in a beaker, and a solution of iron (II) sulphate in a burette. You do not know the concentration of dichromate (VI) ions, but the concentration of the iron (II) solution is known. Your task is to carry out a redox titration using the two solutions in order to determine the concentration of dichromate(VI) ions. Sketch a graph how the e.m.f. changes in the course of above titration. E Cr2O2-7/Cr3+ = 1.33 V, EFe3+/Fe2+ = 0.77 V.a)b)c)d)Correct answer is option 'B'. Can you explain this answer?.
Titrations are one of the methods we can use to discover the precise concentrations of solution. A typical titration involves adding a solution from a burette to another solution in a flask. The endpoint of the titration is found by watching a colour change taking place. However, a problem arises when a suitable indicator cannot be found, or when the colour changes involved are unclear. In cases redox potential may sometimes come to the rescue.A particularly well known example (Fig.1) is a method of discovering the concentration of iron in a solution by titrating them with a solution of cerium (IV). The redox potential that areof interest here are EFe3+/Fe2+ = + 0.77 V and ECe4+/Ce3+ = + 1.61 V. These tell us that cercium (IV) ions are the oxidizing agents, and iron (II) ions are the reducing agent. They should react according to the equationFe2+ (aq) + Ce4+ (aq) Fe3+ (aq) + Ce3+ (aq)Now imagine that we know the concentration of the cerium (IV) ions solution in the burette. We want to measure the concentration of the iron (II) solution. If we add just one drop of the cerium (IV) solution from the bruette, some of the iron (II) ions will be oxidised. As a consequence the beaker would now contain a large number of unreacted ions, but also some iron (III) ions as well. All of the cerium (III). The solution in the beaker now represents an iron(III)/iron(II) half cell, although not at standard conditions. Thus the e.m.f. of the cell will be near, but not equal, to EFe3+/Fe2+.to ad cerium (IV) solution, the number of iron (II) ions is gradually reduced and eventually only a very few are left (Table).At this stage the next few drops of cerium (IV) solution convert all the remaining iron (II) ions into iron (III), and some of the cerium (IV) ions are left unreacted. Once this happens we no longer ions and a smaller number of cerium (IV) ions. The solution in the beaker now behaves as a cerium (IV)/cerium (III) half-cell (although not a standard one).Just before all the iron (II) ions are converted into iron (III) we have a cell with an e.m.f.of around + 0.77 V. After all the iron (II) ions are oxidised, we have a cell with an e.m.f. of about + 1.61 V. This rapid rise in e.m.f. occurs with the addition of hust one drop of cerium (IV) solution. You should be able to understand why a graph of cell e.m.f. against volume ofcerium (IV) solution added looks like that of Fig. 2. The end point of the titration can be read from the graph and the concentration of the iron (II) solution calculated in the usual wayQ.Imagine you were given a solution of potassium dichromate (VI) in a beaker, and a solution of iron (II) sulphate in a burette. You do not know the concentration of dichromate (VI) ions, but the concentration of the iron (II) solution is known. Your task is to carry out a redox titration using the two solutions in order to determine the concentration of dichromate(VI) ions. Sketch a graph how the e.m.f. changes in the course of above titration. E Cr2O2-7/Cr3+ = 1.33 V, EFe3+/Fe2+ = 0.77 V.a)b)c)d)Correct answer is option 'B'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Titrations are one of the methods we can use to discover the precise concentrations of solution. A typical titration involves adding a solution from a burette to another solution in a flask. The endpoint of the titration is found by watching a colour change taking place. However, a problem arises when a suitable indicator cannot be found, or when the colour changes involved are unclear. In cases redox potential may sometimes come to the rescue.A particularly well known example (Fig.1) is a method of discovering the concentration of iron in a solution by titrating them with a solution of cerium (IV). The redox potential that areof interest here are EFe3+/Fe2+ = + 0.77 V and ECe4+/Ce3+ = + 1.61 V. These tell us that cercium (IV) ions are the oxidizing agents, and iron (II) ions are the reducing agent. They should react according to the equationFe2+ (aq) + Ce4+ (aq) Fe3+ (aq) + Ce3+ (aq)Now imagine that we know the concentration of the cerium (IV) ions solution in the burette. We want to measure the concentration of the iron (II) solution. If we add just one drop of the cerium (IV) solution from the bruette, some of the iron (II) ions will be oxidised. As a consequence the beaker would now contain a large number of unreacted ions, but also some iron (III) ions as well. All of the cerium (III). The solution in the beaker now represents an iron(III)/iron(II) half cell, although not at standard conditions. Thus the e.m.f. of the cell will be near, but not equal, to EFe3+/Fe2+.to ad cerium (IV) solution, the number of iron (II) ions is gradually reduced and eventually only a very few are left (Table).At this stage the next few drops of cerium (IV) solution convert all the remaining iron (II) ions into iron (III), and some of the cerium (IV) ions are left unreacted. Once this happens we no longer ions and a smaller number of cerium (IV) ions. The solution in the beaker now behaves as a cerium (IV)/cerium (III) half-cell (although not a standard one).Just before all the iron (II) ions are converted into iron (III) we have a cell with an e.m.f.of around + 0.77 V. After all the iron (II) ions are oxidised, we have a cell with an e.m.f. of about + 1.61 V. This rapid rise in e.m.f. occurs with the addition of hust one drop of cerium (IV) solution. You should be able to understand why a graph of cell e.m.f. against volume ofcerium (IV) solution added looks like that of Fig. 2. The end point of the titration can be read from the graph and the concentration of the iron (II) solution calculated in the usual wayQ.Imagine you were given a solution of potassium dichromate (VI) in a beaker, and a solution of iron (II) sulphate in a burette. You do not know the concentration of dichromate (VI) ions, but the concentration of the iron (II) solution is known. Your task is to carry out a redox titration using the two solutions in order to determine the concentration of dichromate(VI) ions. Sketch a graph how the e.m.f. changes in the course of above titration. E Cr2O2-7/Cr3+ = 1.33 V, EFe3+/Fe2+ = 0.77 V.a)b)c)d)Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Titrations are one of the methods we can use to discover the precise concentrations of solution. A typical titration involves adding a solution from a burette to another solution in a flask. The endpoint of the titration is found by watching a colour change taking place. However, a problem arises when a suitable indicator cannot be found, or when the colour changes involved are unclear. In cases redox potential may sometimes come to the rescue.A particularly well known example (Fig.1) is a method of discovering the concentration of iron in a solution by titrating them with a solution of cerium (IV). The redox potential that areof interest here are EFe3+/Fe2+ = + 0.77 V and ECe4+/Ce3+ = + 1.61 V. These tell us that cercium (IV) ions are the oxidizing agents, and iron (II) ions are the reducing agent. They should react according to the equationFe2+ (aq) + Ce4+ (aq) Fe3+ (aq) + Ce3+ (aq)Now imagine that we know the concentration of the cerium (IV) ions solution in the burette. We want to measure the concentration of the iron (II) solution. If we add just one drop of the cerium (IV) solution from the bruette, some of the iron (II) ions will be oxidised. As a consequence the beaker would now contain a large number of unreacted ions, but also some iron (III) ions as well. All of the cerium (III). The solution in the beaker now represents an iron(III)/iron(II) half cell, although not at standard conditions. Thus the e.m.f. of the cell will be near, but not equal, to EFe3+/Fe2+.to ad cerium (IV) solution, the number of iron (II) ions is gradually reduced and eventually only a very few are left (Table).At this stage the next few drops of cerium (IV) solution convert all the remaining iron (II) ions into iron (III), and some of the cerium (IV) ions are left unreacted. Once this happens we no longer ions and a smaller number of cerium (IV) ions. The solution in the beaker now behaves as a cerium (IV)/cerium (III) half-cell (although not a standard one).Just before all the iron (II) ions are converted into iron (III) we have a cell with an e.m.f.of around + 0.77 V. After all the iron (II) ions are oxidised, we have a cell with an e.m.f. of about + 1.61 V. This rapid rise in e.m.f. occurs with the addition of hust one drop of cerium (IV) solution. You should be able to understand why a graph of cell e.m.f. against volume ofcerium (IV) solution added looks like that of Fig. 2. The end point of the titration can be read from the graph and the concentration of the iron (II) solution calculated in the usual wayQ.Imagine you were given a solution of potassium dichromate (VI) in a beaker, and a solution of iron (II) sulphate in a burette. You do not know the concentration of dichromate (VI) ions, but the concentration of the iron (II) solution is known. Your task is to carry out a redox titration using the two solutions in order to determine the concentration of dichromate(VI) ions. Sketch a graph how the e.m.f. changes in the course of above titration. E Cr2O2-7/Cr3+ = 1.33 V, EFe3+/Fe2+ = 0.77 V.a)b)c)d)Correct answer is option 'B'. Can you explain this answer?.
Solutions for Titrations are one of the methods we can use to discover the precise concentrations of solution. A typical titration involves adding a solution from a burette to another solution in a flask. The endpoint of the titration is found by watching a colour change taking place. However, a problem arises when a suitable indicator cannot be found, or when the colour changes involved are unclear. In cases redox potential may sometimes come to the rescue.A particularly well known example (Fig.1) is a method of discovering the concentration of iron in a solution by titrating them with a solution of cerium (IV). The redox potential that areof interest here are EFe3+/Fe2+ = + 0.77 V and ECe4+/Ce3+ = + 1.61 V. These tell us that cercium (IV) ions are the oxidizing agents, and iron (II) ions are the reducing agent. They should react according to the equationFe2+ (aq) + Ce4+ (aq) Fe3+ (aq) + Ce3+ (aq)Now imagine that we know the concentration of the cerium (IV) ions solution in the burette. We want to measure the concentration of the iron (II) solution. If we add just one drop of the cerium (IV) solution from the bruette, some of the iron (II) ions will be oxidised. As a consequence the beaker would now contain a large number of unreacted ions, but also some iron (III) ions as well. All of the cerium (III). The solution in the beaker now represents an iron(III)/iron(II) half cell, although not at standard conditions. Thus the e.m.f. of the cell will be near, but not equal, to EFe3+/Fe2+.to ad cerium (IV) solution, the number of iron (II) ions is gradually reduced and eventually only a very few are left (Table).At this stage the next few drops of cerium (IV) solution convert all the remaining iron (II) ions into iron (III), and some of the cerium (IV) ions are left unreacted. Once this happens we no longer ions and a smaller number of cerium (IV) ions. The solution in the beaker now behaves as a cerium (IV)/cerium (III) half-cell (although not a standard one).Just before all the iron (II) ions are converted into iron (III) we have a cell with an e.m.f.of around + 0.77 V. After all the iron (II) ions are oxidised, we have a cell with an e.m.f. of about + 1.61 V. This rapid rise in e.m.f. occurs with the addition of hust one drop of cerium (IV) solution. You should be able to understand why a graph of cell e.m.f. against volume ofcerium (IV) solution added looks like that of Fig. 2. The end point of the titration can be read from the graph and the concentration of the iron (II) solution calculated in the usual wayQ.Imagine you were given a solution of potassium dichromate (VI) in a beaker, and a solution of iron (II) sulphate in a burette. You do not know the concentration of dichromate (VI) ions, but the concentration of the iron (II) solution is known. Your task is to carry out a redox titration using the two solutions in order to determine the concentration of dichromate(VI) ions. Sketch a graph how the e.m.f. changes in the course of above titration. E Cr2O2-7/Cr3+ = 1.33 V, EFe3+/Fe2+ = 0.77 V.a)b)c)d)Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Titrations are one of the methods we can use to discover the precise concentrations of solution. A typical titration involves adding a solution from a burette to another solution in a flask. The endpoint of the titration is found by watching a colour change taking place. However, a problem arises when a suitable indicator cannot be found, or when the colour changes involved are unclear. In cases redox potential may sometimes come to the rescue.A particularly well known example (Fig.1) is a method of discovering the concentration of iron in a solution by titrating them with a solution of cerium (IV). The redox potential that areof interest here are EFe3+/Fe2+ = + 0.77 V and ECe4+/Ce3+ = + 1.61 V. These tell us that cercium (IV) ions are the oxidizing agents, and iron (II) ions are the reducing agent. They should react according to the equationFe2+ (aq) + Ce4+ (aq) Fe3+ (aq) + Ce3+ (aq)Now imagine that we know the concentration of the cerium (IV) ions solution in the burette. We want to measure the concentration of the iron (II) solution. If we add just one drop of the cerium (IV) solution from the bruette, some of the iron (II) ions will be oxidised. As a consequence the beaker would now contain a large number of unreacted ions, but also some iron (III) ions as well. All of the cerium (III). The solution in the beaker now represents an iron(III)/iron(II) half cell, although not at standard conditions. Thus the e.m.f. of the cell will be near, but not equal, to EFe3+/Fe2+.to ad cerium (IV) solution, the number of iron (II) ions is gradually reduced and eventually only a very few are left (Table).At this stage the next few drops of cerium (IV) solution convert all the remaining iron (II) ions into iron (III), and some of the cerium (IV) ions are left unreacted. Once this happens we no longer ions and a smaller number of cerium (IV) ions. The solution in the beaker now behaves as a cerium (IV)/cerium (III) half-cell (although not a standard one).Just before all the iron (II) ions are converted into iron (III) we have a cell with an e.m.f.of around + 0.77 V. After all the iron (II) ions are oxidised, we have a cell with an e.m.f. of about + 1.61 V. This rapid rise in e.m.f. occurs with the addition of hust one drop of cerium (IV) solution. You should be able to understand why a graph of cell e.m.f. against volume ofcerium (IV) solution added looks like that of Fig. 2. The end point of the titration can be read from the graph and the concentration of the iron (II) solution calculated in the usual wayQ.Imagine you were given a solution of potassium dichromate (VI) in a beaker, and a solution of iron (II) sulphate in a burette. You do not know the concentration of dichromate (VI) ions, but the concentration of the iron (II) solution is known. Your task is to carry out a redox titration using the two solutions in order to determine the concentration of dichromate(VI) ions. Sketch a graph how the e.m.f. changes in the course of above titration. E Cr2O2-7/Cr3+ = 1.33 V, EFe3+/Fe2+ = 0.77 V.a)b)c)d)Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Titrations are one of the methods we can use to discover the precise concentrations of solution. A typical titration involves adding a solution from a burette to another solution in a flask. The endpoint of the titration is found by watching a colour change taking place. However, a problem arises when a suitable indicator cannot be found, or when the colour changes involved are unclear. In cases redox potential may sometimes come to the rescue.A particularly well known example (Fig.1) is a method of discovering the concentration of iron in a solution by titrating them with a solution of cerium (IV). The redox potential that areof interest here are EFe3+/Fe2+ = + 0.77 V and ECe4+/Ce3+ = + 1.61 V. These tell us that cercium (IV) ions are the oxidizing agents, and iron (II) ions are the reducing agent. They should react according to the equationFe2+ (aq) + Ce4+ (aq) Fe3+ (aq) + Ce3+ (aq)Now imagine that we know the concentration of the cerium (IV) ions solution in the burette. We want to measure the concentration of the iron (II) solution. If we add just one drop of the cerium (IV) solution from the bruette, some of the iron (II) ions will be oxidised. As a consequence the beaker would now contain a large number of unreacted ions, but also some iron (III) ions as well. All of the cerium (III). The solution in the beaker now represents an iron(III)/iron(II) half cell, although not at standard conditions. Thus the e.m.f. of the cell will be near, but not equal, to EFe3+/Fe2+.to ad cerium (IV) solution, the number of iron (II) ions is gradually reduced and eventually only a very few are left (Table).At this stage the next few drops of cerium (IV) solution convert all the remaining iron (II) ions into iron (III), and some of the cerium (IV) ions are left unreacted. Once this happens we no longer ions and a smaller number of cerium (IV) ions. The solution in the beaker now behaves as a cerium (IV)/cerium (III) half-cell (although not a standard one).Just before all the iron (II) ions are converted into iron (III) we have a cell with an e.m.f.of around + 0.77 V. After all the iron (II) ions are oxidised, we have a cell with an e.m.f. of about + 1.61 V. This rapid rise in e.m.f. occurs with the addition of hust one drop of cerium (IV) solution. You should be able to understand why a graph of cell e.m.f. against volume ofcerium (IV) solution added looks like that of Fig. 2. The end point of the titration can be read from the graph and the concentration of the iron (II) solution calculated in the usual wayQ.Imagine you were given a solution of potassium dichromate (VI) in a beaker, and a solution of iron (II) sulphate in a burette. You do not know the concentration of dichromate (VI) ions, but the concentration of the iron (II) solution is known. Your task is to carry out a redox titration using the two solutions in order to determine the concentration of dichromate(VI) ions. Sketch a graph how the e.m.f. changes in the course of above titration. E Cr2O2-7/Cr3+ = 1.33 V, EFe3+/Fe2+ = 0.77 V.a)b)c)d)Correct answer is option 'B'. Can you explain this answer?, a detailed solution for Titrations are one of the methods we can use to discover the precise concentrations of solution. A typical titration involves adding a solution from a burette to another solution in a flask. The endpoint of the titration is found by watching a colour change taking place. However, a problem arises when a suitable indicator cannot be found, or when the colour changes involved are unclear. In cases redox potential may sometimes come to the rescue.A particularly well known example (Fig.1) is a method of discovering the concentration of iron in a solution by titrating them with a solution of cerium (IV). The redox potential that areof interest here are EFe3+/Fe2+ = + 0.77 V and ECe4+/Ce3+ = + 1.61 V. These tell us that cercium (IV) ions are the oxidizing agents, and iron (II) ions are the reducing agent. They should react according to the equationFe2+ (aq) + Ce4+ (aq) Fe3+ (aq) + Ce3+ (aq)Now imagine that we know the concentration of the cerium (IV) ions solution in the burette. We want to measure the concentration of the iron (II) solution. If we add just one drop of the cerium (IV) solution from the bruette, some of the iron (II) ions will be oxidised. As a consequence the beaker would now contain a large number of unreacted ions, but also some iron (III) ions as well. All of the cerium (III). The solution in the beaker now represents an iron(III)/iron(II) half cell, although not at standard conditions. Thus the e.m.f. of the cell will be near, but not equal, to EFe3+/Fe2+.to ad cerium (IV) solution, the number of iron (II) ions is gradually reduced and eventually only a very few are left (Table).At this stage the next few drops of cerium (IV) solution convert all the remaining iron (II) ions into iron (III), and some of the cerium (IV) ions are left unreacted. Once this happens we no longer ions and a smaller number of cerium (IV) ions. The solution in the beaker now behaves as a cerium (IV)/cerium (III) half-cell (although not a standard one).Just before all the iron (II) ions are converted into iron (III) we have a cell with an e.m.f.of around + 0.77 V. After all the iron (II) ions are oxidised, we have a cell with an e.m.f. of about + 1.61 V. This rapid rise in e.m.f. occurs with the addition of hust one drop of cerium (IV) solution. You should be able to understand why a graph of cell e.m.f. against volume ofcerium (IV) solution added looks like that of Fig. 2. The end point of the titration can be read from the graph and the concentration of the iron (II) solution calculated in the usual wayQ.Imagine you were given a solution of potassium dichromate (VI) in a beaker, and a solution of iron (II) sulphate in a burette. You do not know the concentration of dichromate (VI) ions, but the concentration of the iron (II) solution is known. Your task is to carry out a redox titration using the two solutions in order to determine the concentration of dichromate(VI) ions. Sketch a graph how the e.m.f. changes in the course of above titration. E Cr2O2-7/Cr3+ = 1.33 V, EFe3+/Fe2+ = 0.77 V.a)b)c)d)Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of Titrations are one of the methods we can use to discover the precise concentrations of solution. A typical titration involves adding a solution from a burette to another solution in a flask. The endpoint of the titration is found by watching a colour change taking place. However, a problem arises when a suitable indicator cannot be found, or when the colour changes involved are unclear. In cases redox potential may sometimes come to the rescue.A particularly well known example (Fig.1) is a method of discovering the concentration of iron in a solution by titrating them with a solution of cerium (IV). The redox potential that areof interest here are EFe3+/Fe2+ = + 0.77 V and ECe4+/Ce3+ = + 1.61 V. These tell us that cercium (IV) ions are the oxidizing agents, and iron (II) ions are the reducing agent. They should react according to the equationFe2+ (aq) + Ce4+ (aq) Fe3+ (aq) + Ce3+ (aq)Now imagine that we know the concentration of the cerium (IV) ions solution in the burette. We want to measure the concentration of the iron (II) solution. If we add just one drop of the cerium (IV) solution from the bruette, some of the iron (II) ions will be oxidised. As a consequence the beaker would now contain a large number of unreacted ions, but also some iron (III) ions as well. All of the cerium (III). The solution in the beaker now represents an iron(III)/iron(II) half cell, although not at standard conditions. Thus the e.m.f. of the cell will be near, but not equal, to EFe3+/Fe2+.to ad cerium (IV) solution, the number of iron (II) ions is gradually reduced and eventually only a very few are left (Table).At this stage the next few drops of cerium (IV) solution convert all the remaining iron (II) ions into iron (III), and some of the cerium (IV) ions are left unreacted. Once this happens we no longer ions and a smaller number of cerium (IV) ions. The solution in the beaker now behaves as a cerium (IV)/cerium (III) half-cell (although not a standard one).Just before all the iron (II) ions are converted into iron (III) we have a cell with an e.m.f.of around + 0.77 V. After all the iron (II) ions are oxidised, we have a cell with an e.m.f. of about + 1.61 V. This rapid rise in e.m.f. occurs with the addition of hust one drop of cerium (IV) solution. You should be able to understand why a graph of cell e.m.f. against volume ofcerium (IV) solution added looks like that of Fig. 2. The end point of the titration can be read from the graph and the concentration of the iron (II) solution calculated in the usual wayQ.Imagine you were given a solution of potassium dichromate (VI) in a beaker, and a solution of iron (II) sulphate in a burette. You do not know the concentration of dichromate (VI) ions, but the concentration of the iron (II) solution is known. Your task is to carry out a redox titration using the two solutions in order to determine the concentration of dichromate(VI) ions. Sketch a graph how the e.m.f. changes in the course of above titration. E Cr2O2-7/Cr3+ = 1.33 V, EFe3+/Fe2+ = 0.77 V.a)b)c)d)Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Titrations are one of the methods we can use to discover the precise concentrations of solution. A typical titration involves adding a solution from a burette to another solution in a flask. The endpoint of the titration is found by watching a colour change taking place. However, a problem arises when a suitable indicator cannot be found, or when the colour changes involved are unclear. In cases redox potential may sometimes come to the rescue.A particularly well known example (Fig.1) is a method of discovering the concentration of iron in a solution by titrating them with a solution of cerium (IV). The redox potential that areof interest here are EFe3+/Fe2+ = + 0.77 V and ECe4+/Ce3+ = + 1.61 V. These tell us that cercium (IV) ions are the oxidizing agents, and iron (II) ions are the reducing agent. They should react according to the equationFe2+ (aq) + Ce4+ (aq) Fe3+ (aq) + Ce3+ (aq)Now imagine that we know the concentration of the cerium (IV) ions solution in the burette. We want to measure the concentration of the iron (II) solution. If we add just one drop of the cerium (IV) solution from the bruette, some of the iron (II) ions will be oxidised. As a consequence the beaker would now contain a large number of unreacted ions, but also some iron (III) ions as well. All of the cerium (III). The solution in the beaker now represents an iron(III)/iron(II) half cell, although not at standard conditions. Thus the e.m.f. of the cell will be near, but not equal, to EFe3+/Fe2+.to ad cerium (IV) solution, the number of iron (II) ions is gradually reduced and eventually only a very few are left (Table).At this stage the next few drops of cerium (IV) solution convert all the remaining iron (II) ions into iron (III), and some of the cerium (IV) ions are left unreacted. Once this happens we no longer ions and a smaller number of cerium (IV) ions. The solution in the beaker now behaves as a cerium (IV)/cerium (III) half-cell (although not a standard one).Just before all the iron (II) ions are converted into iron (III) we have a cell with an e.m.f.of around + 0.77 V. After all the iron (II) ions are oxidised, we have a cell with an e.m.f. of about + 1.61 V. This rapid rise in e.m.f. occurs with the addition of hust one drop of cerium (IV) solution. You should be able to understand why a graph of cell e.m.f. against volume ofcerium (IV) solution added looks like that of Fig. 2. The end point of the titration can be read from the graph and the concentration of the iron (II) solution calculated in the usual wayQ.Imagine you were given a solution of potassium dichromate (VI) in a beaker, and a solution of iron (II) sulphate in a burette. You do not know the concentration of dichromate (VI) ions, but the concentration of the iron (II) solution is known. Your task is to carry out a redox titration using the two solutions in order to determine the concentration of dichromate(VI) ions. Sketch a graph how the e.m.f. changes in the course of above titration. E Cr2O2-7/Cr3+ = 1.33 V, EFe3+/Fe2+ = 0.77 V.a)b)c)d)Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























