JEE Exam > JEE Questions > Consider a solution of CH3COONH4 which is a s...
Start Learning for Free
Consider a solution of CH3COONH4 which is a salt of weak acid &weak base. The
equilibrium involved in the solutions are :
If we add these three reactions, then the net reaction is
Both CH3C00- and NH4' get hydrolysed independently and their hydrolysis depends on
(i) their initial concentration
(ii) the value of Kh which is 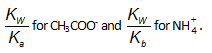
Since both of the ions were produced from the same salt, their initial concentrations are same . Therefore unless
& untial the value of Kw/Ka and Kb is same, the degree of hydrolysis of ion can't be same.
& untial the value of Kw/Ka and Kb is same, the degree of hydrolysis of ion can't be same.
To explain why we assume that degree of hydrolysis of cation and anion is same, we need to now look at the third reaction i.e., combination of H+ and OH – ions. It is obvious that this reaction happens only because one reaction produced H+ ion and the other produced OH– ions. We can also note that this reaction causes both the hydrolysis reaction to occur more since their product ions are being consumed. Keep this thing in mind that the equilibrium which has smaller value of equilibrium conxtant is affected more by the common ion effect. For the same reason if for any reason a reaction is made to occur to a greater extent by the consumption of any one of the product ion, the reaction with the smaller value of equilibrium constant tends to get affected more.
Therefore we conclude that firstly the hydrolysis of both the ions ocurs more in the presence of each other (due to consumption of the product ions) than in each other is absence. Secondly the hydrolysis of the ion which occurs to a lesser extent (due to smaller value of Kh) is affected more than the one whose Kh is greater. Hence we can see that the degree of hydrolysis of both the ions would be close to each other when they are getting hydrolysed in the presence of each other.
Q.
In a solution of NaHCO3 , the amphiprotic anion can under ionization to form H+ ion and hydrolysis to from OH– ion.
To calculat PH, suitable approximation is :
- a)[CO32-] = [HCO3]
- b)degree of ionization = degree of Hydrolysis
- c)both (A) and (B)
- d)neither 'A' nore 'B'
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Consider a solution of CH3COONH4 which is a salt of weak acid weak bas...

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Consider a solution of CH3COONH4 which is a salt of weak acid weak base. Theequilibrium involved in the solutions are :If we add these three reactions, then the net reaction isBoth CH3C00- and NH4 get hydrolysed independently and their hydrolysis depends on(i) their initial concentration(ii) the value of Kh which is Since both of the ions were produced from the same salt, their initial concentrations are same . Therefore unless untial the value of Kw/Kaand Kb is same, the degree of hydrolysis of ion cant be same.To explain why we assume that degree of hydrolysis of cation and anion is same, we need to now look at the third reaction i.e., combination of H+ and OH ions. It is obvious that this reaction happens only because one reaction produced H+ ion and the other produced OH ions. We can also note that this reaction causes both the hydrolysis reaction to occur more since their product ions are being consumed. Keep this thing in mind that the equilibrium which has smaller value of equilibrium conxtant is affected more by the common ion effect. For the same reason if for any reason a reaction is made to occur to a greater extent by the consumption of any one of the product ion, the reaction with the smaller value of equilibrium constant tends to get affected more.Therefore we conclude that firstly the hydrolysis of both the ions ocurs more in the presence of each other (due to consumption of the product ions) than in each other is absence. Secondly the hydrolysis of the ion which occurs to a lesser extent (due to smaller value of Kh) is affected more than the one whose Kh is greater. Hence we can see that the degree of hydrolysis of both the ions would be close to each other when they are getting hydrolysed in the presence of each other.Q.In a solution of NaHCO3 , the amphiprotic anion can under ionization to form H+ ion and hydrolysis to from OH ion.To calculat PH, suitable approximation is :a)[CO32-] = [HCO3]b)degree of ionization = degree of Hydrolysisc)both (A) and (B)d)neither A nore BCorrect answer is option 'C'. Can you explain this answer?
Question Description
Consider a solution of CH3COONH4 which is a salt of weak acid weak base. Theequilibrium involved in the solutions are :If we add these three reactions, then the net reaction isBoth CH3C00- and NH4 get hydrolysed independently and their hydrolysis depends on(i) their initial concentration(ii) the value of Kh which is Since both of the ions were produced from the same salt, their initial concentrations are same . Therefore unless untial the value of Kw/Kaand Kb is same, the degree of hydrolysis of ion cant be same.To explain why we assume that degree of hydrolysis of cation and anion is same, we need to now look at the third reaction i.e., combination of H+ and OH ions. It is obvious that this reaction happens only because one reaction produced H+ ion and the other produced OH ions. We can also note that this reaction causes both the hydrolysis reaction to occur more since their product ions are being consumed. Keep this thing in mind that the equilibrium which has smaller value of equilibrium conxtant is affected more by the common ion effect. For the same reason if for any reason a reaction is made to occur to a greater extent by the consumption of any one of the product ion, the reaction with the smaller value of equilibrium constant tends to get affected more.Therefore we conclude that firstly the hydrolysis of both the ions ocurs more in the presence of each other (due to consumption of the product ions) than in each other is absence. Secondly the hydrolysis of the ion which occurs to a lesser extent (due to smaller value of Kh) is affected more than the one whose Kh is greater. Hence we can see that the degree of hydrolysis of both the ions would be close to each other when they are getting hydrolysed in the presence of each other.Q.In a solution of NaHCO3 , the amphiprotic anion can under ionization to form H+ ion and hydrolysis to from OH ion.To calculat PH, suitable approximation is :a)[CO32-] = [HCO3]b)degree of ionization = degree of Hydrolysisc)both (A) and (B)d)neither A nore BCorrect answer is option 'C'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Consider a solution of CH3COONH4 which is a salt of weak acid weak base. Theequilibrium involved in the solutions are :If we add these three reactions, then the net reaction isBoth CH3C00- and NH4 get hydrolysed independently and their hydrolysis depends on(i) their initial concentration(ii) the value of Kh which is Since both of the ions were produced from the same salt, their initial concentrations are same . Therefore unless untial the value of Kw/Kaand Kb is same, the degree of hydrolysis of ion cant be same.To explain why we assume that degree of hydrolysis of cation and anion is same, we need to now look at the third reaction i.e., combination of H+ and OH ions. It is obvious that this reaction happens only because one reaction produced H+ ion and the other produced OH ions. We can also note that this reaction causes both the hydrolysis reaction to occur more since their product ions are being consumed. Keep this thing in mind that the equilibrium which has smaller value of equilibrium conxtant is affected more by the common ion effect. For the same reason if for any reason a reaction is made to occur to a greater extent by the consumption of any one of the product ion, the reaction with the smaller value of equilibrium constant tends to get affected more.Therefore we conclude that firstly the hydrolysis of both the ions ocurs more in the presence of each other (due to consumption of the product ions) than in each other is absence. Secondly the hydrolysis of the ion which occurs to a lesser extent (due to smaller value of Kh) is affected more than the one whose Kh is greater. Hence we can see that the degree of hydrolysis of both the ions would be close to each other when they are getting hydrolysed in the presence of each other.Q.In a solution of NaHCO3 , the amphiprotic anion can under ionization to form H+ ion and hydrolysis to from OH ion.To calculat PH, suitable approximation is :a)[CO32-] = [HCO3]b)degree of ionization = degree of Hydrolysisc)both (A) and (B)d)neither A nore BCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Consider a solution of CH3COONH4 which is a salt of weak acid weak base. Theequilibrium involved in the solutions are :If we add these three reactions, then the net reaction isBoth CH3C00- and NH4 get hydrolysed independently and their hydrolysis depends on(i) their initial concentration(ii) the value of Kh which is Since both of the ions were produced from the same salt, their initial concentrations are same . Therefore unless untial the value of Kw/Kaand Kb is same, the degree of hydrolysis of ion cant be same.To explain why we assume that degree of hydrolysis of cation and anion is same, we need to now look at the third reaction i.e., combination of H+ and OH ions. It is obvious that this reaction happens only because one reaction produced H+ ion and the other produced OH ions. We can also note that this reaction causes both the hydrolysis reaction to occur more since their product ions are being consumed. Keep this thing in mind that the equilibrium which has smaller value of equilibrium conxtant is affected more by the common ion effect. For the same reason if for any reason a reaction is made to occur to a greater extent by the consumption of any one of the product ion, the reaction with the smaller value of equilibrium constant tends to get affected more.Therefore we conclude that firstly the hydrolysis of both the ions ocurs more in the presence of each other (due to consumption of the product ions) than in each other is absence. Secondly the hydrolysis of the ion which occurs to a lesser extent (due to smaller value of Kh) is affected more than the one whose Kh is greater. Hence we can see that the degree of hydrolysis of both the ions would be close to each other when they are getting hydrolysed in the presence of each other.Q.In a solution of NaHCO3 , the amphiprotic anion can under ionization to form H+ ion and hydrolysis to from OH ion.To calculat PH, suitable approximation is :a)[CO32-] = [HCO3]b)degree of ionization = degree of Hydrolysisc)both (A) and (B)d)neither A nore BCorrect answer is option 'C'. Can you explain this answer?.
Consider a solution of CH3COONH4 which is a salt of weak acid weak base. Theequilibrium involved in the solutions are :If we add these three reactions, then the net reaction isBoth CH3C00- and NH4 get hydrolysed independently and their hydrolysis depends on(i) their initial concentration(ii) the value of Kh which is Since both of the ions were produced from the same salt, their initial concentrations are same . Therefore unless untial the value of Kw/Kaand Kb is same, the degree of hydrolysis of ion cant be same.To explain why we assume that degree of hydrolysis of cation and anion is same, we need to now look at the third reaction i.e., combination of H+ and OH ions. It is obvious that this reaction happens only because one reaction produced H+ ion and the other produced OH ions. We can also note that this reaction causes both the hydrolysis reaction to occur more since their product ions are being consumed. Keep this thing in mind that the equilibrium which has smaller value of equilibrium conxtant is affected more by the common ion effect. For the same reason if for any reason a reaction is made to occur to a greater extent by the consumption of any one of the product ion, the reaction with the smaller value of equilibrium constant tends to get affected more.Therefore we conclude that firstly the hydrolysis of both the ions ocurs more in the presence of each other (due to consumption of the product ions) than in each other is absence. Secondly the hydrolysis of the ion which occurs to a lesser extent (due to smaller value of Kh) is affected more than the one whose Kh is greater. Hence we can see that the degree of hydrolysis of both the ions would be close to each other when they are getting hydrolysed in the presence of each other.Q.In a solution of NaHCO3 , the amphiprotic anion can under ionization to form H+ ion and hydrolysis to from OH ion.To calculat PH, suitable approximation is :a)[CO32-] = [HCO3]b)degree of ionization = degree of Hydrolysisc)both (A) and (B)d)neither A nore BCorrect answer is option 'C'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Consider a solution of CH3COONH4 which is a salt of weak acid weak base. Theequilibrium involved in the solutions are :If we add these three reactions, then the net reaction isBoth CH3C00- and NH4 get hydrolysed independently and their hydrolysis depends on(i) their initial concentration(ii) the value of Kh which is Since both of the ions were produced from the same salt, their initial concentrations are same . Therefore unless untial the value of Kw/Kaand Kb is same, the degree of hydrolysis of ion cant be same.To explain why we assume that degree of hydrolysis of cation and anion is same, we need to now look at the third reaction i.e., combination of H+ and OH ions. It is obvious that this reaction happens only because one reaction produced H+ ion and the other produced OH ions. We can also note that this reaction causes both the hydrolysis reaction to occur more since their product ions are being consumed. Keep this thing in mind that the equilibrium which has smaller value of equilibrium conxtant is affected more by the common ion effect. For the same reason if for any reason a reaction is made to occur to a greater extent by the consumption of any one of the product ion, the reaction with the smaller value of equilibrium constant tends to get affected more.Therefore we conclude that firstly the hydrolysis of both the ions ocurs more in the presence of each other (due to consumption of the product ions) than in each other is absence. Secondly the hydrolysis of the ion which occurs to a lesser extent (due to smaller value of Kh) is affected more than the one whose Kh is greater. Hence we can see that the degree of hydrolysis of both the ions would be close to each other when they are getting hydrolysed in the presence of each other.Q.In a solution of NaHCO3 , the amphiprotic anion can under ionization to form H+ ion and hydrolysis to from OH ion.To calculat PH, suitable approximation is :a)[CO32-] = [HCO3]b)degree of ionization = degree of Hydrolysisc)both (A) and (B)d)neither A nore BCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Consider a solution of CH3COONH4 which is a salt of weak acid weak base. Theequilibrium involved in the solutions are :If we add these three reactions, then the net reaction isBoth CH3C00- and NH4 get hydrolysed independently and their hydrolysis depends on(i) their initial concentration(ii) the value of Kh which is Since both of the ions were produced from the same salt, their initial concentrations are same . Therefore unless untial the value of Kw/Kaand Kb is same, the degree of hydrolysis of ion cant be same.To explain why we assume that degree of hydrolysis of cation and anion is same, we need to now look at the third reaction i.e., combination of H+ and OH ions. It is obvious that this reaction happens only because one reaction produced H+ ion and the other produced OH ions. We can also note that this reaction causes both the hydrolysis reaction to occur more since their product ions are being consumed. Keep this thing in mind that the equilibrium which has smaller value of equilibrium conxtant is affected more by the common ion effect. For the same reason if for any reason a reaction is made to occur to a greater extent by the consumption of any one of the product ion, the reaction with the smaller value of equilibrium constant tends to get affected more.Therefore we conclude that firstly the hydrolysis of both the ions ocurs more in the presence of each other (due to consumption of the product ions) than in each other is absence. Secondly the hydrolysis of the ion which occurs to a lesser extent (due to smaller value of Kh) is affected more than the one whose Kh is greater. Hence we can see that the degree of hydrolysis of both the ions would be close to each other when they are getting hydrolysed in the presence of each other.Q.In a solution of NaHCO3 , the amphiprotic anion can under ionization to form H+ ion and hydrolysis to from OH ion.To calculat PH, suitable approximation is :a)[CO32-] = [HCO3]b)degree of ionization = degree of Hydrolysisc)both (A) and (B)d)neither A nore BCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Consider a solution of CH3COONH4 which is a salt of weak acid weak base. Theequilibrium involved in the solutions are :If we add these three reactions, then the net reaction isBoth CH3C00- and NH4 get hydrolysed independently and their hydrolysis depends on(i) their initial concentration(ii) the value of Kh which is Since both of the ions were produced from the same salt, their initial concentrations are same . Therefore unless untial the value of Kw/Kaand Kb is same, the degree of hydrolysis of ion cant be same.To explain why we assume that degree of hydrolysis of cation and anion is same, we need to now look at the third reaction i.e., combination of H+ and OH ions. It is obvious that this reaction happens only because one reaction produced H+ ion and the other produced OH ions. We can also note that this reaction causes both the hydrolysis reaction to occur more since their product ions are being consumed. Keep this thing in mind that the equilibrium which has smaller value of equilibrium conxtant is affected more by the common ion effect. For the same reason if for any reason a reaction is made to occur to a greater extent by the consumption of any one of the product ion, the reaction with the smaller value of equilibrium constant tends to get affected more.Therefore we conclude that firstly the hydrolysis of both the ions ocurs more in the presence of each other (due to consumption of the product ions) than in each other is absence. Secondly the hydrolysis of the ion which occurs to a lesser extent (due to smaller value of Kh) is affected more than the one whose Kh is greater. Hence we can see that the degree of hydrolysis of both the ions would be close to each other when they are getting hydrolysed in the presence of each other.Q.In a solution of NaHCO3 , the amphiprotic anion can under ionization to form H+ ion and hydrolysis to from OH ion.To calculat PH, suitable approximation is :a)[CO32-] = [HCO3]b)degree of ionization = degree of Hydrolysisc)both (A) and (B)d)neither A nore BCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Consider a solution of CH3COONH4 which is a salt of weak acid weak base. Theequilibrium involved in the solutions are :If we add these three reactions, then the net reaction isBoth CH3C00- and NH4 get hydrolysed independently and their hydrolysis depends on(i) their initial concentration(ii) the value of Kh which is Since both of the ions were produced from the same salt, their initial concentrations are same . Therefore unless untial the value of Kw/Kaand Kb is same, the degree of hydrolysis of ion cant be same.To explain why we assume that degree of hydrolysis of cation and anion is same, we need to now look at the third reaction i.e., combination of H+ and OH ions. It is obvious that this reaction happens only because one reaction produced H+ ion and the other produced OH ions. We can also note that this reaction causes both the hydrolysis reaction to occur more since their product ions are being consumed. Keep this thing in mind that the equilibrium which has smaller value of equilibrium conxtant is affected more by the common ion effect. For the same reason if for any reason a reaction is made to occur to a greater extent by the consumption of any one of the product ion, the reaction with the smaller value of equilibrium constant tends to get affected more.Therefore we conclude that firstly the hydrolysis of both the ions ocurs more in the presence of each other (due to consumption of the product ions) than in each other is absence. Secondly the hydrolysis of the ion which occurs to a lesser extent (due to smaller value of Kh) is affected more than the one whose Kh is greater. Hence we can see that the degree of hydrolysis of both the ions would be close to each other when they are getting hydrolysed in the presence of each other.Q.In a solution of NaHCO3 , the amphiprotic anion can under ionization to form H+ ion and hydrolysis to from OH ion.To calculat PH, suitable approximation is :a)[CO32-] = [HCO3]b)degree of ionization = degree of Hydrolysisc)both (A) and (B)d)neither A nore BCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Consider a solution of CH3COONH4 which is a salt of weak acid weak base. Theequilibrium involved in the solutions are :If we add these three reactions, then the net reaction isBoth CH3C00- and NH4 get hydrolysed independently and their hydrolysis depends on(i) their initial concentration(ii) the value of Kh which is Since both of the ions were produced from the same salt, their initial concentrations are same . Therefore unless untial the value of Kw/Kaand Kb is same, the degree of hydrolysis of ion cant be same.To explain why we assume that degree of hydrolysis of cation and anion is same, we need to now look at the third reaction i.e., combination of H+ and OH ions. It is obvious that this reaction happens only because one reaction produced H+ ion and the other produced OH ions. We can also note that this reaction causes both the hydrolysis reaction to occur more since their product ions are being consumed. Keep this thing in mind that the equilibrium which has smaller value of equilibrium conxtant is affected more by the common ion effect. For the same reason if for any reason a reaction is made to occur to a greater extent by the consumption of any one of the product ion, the reaction with the smaller value of equilibrium constant tends to get affected more.Therefore we conclude that firstly the hydrolysis of both the ions ocurs more in the presence of each other (due to consumption of the product ions) than in each other is absence. Secondly the hydrolysis of the ion which occurs to a lesser extent (due to smaller value of Kh) is affected more than the one whose Kh is greater. Hence we can see that the degree of hydrolysis of both the ions would be close to each other when they are getting hydrolysed in the presence of each other.Q.In a solution of NaHCO3 , the amphiprotic anion can under ionization to form H+ ion and hydrolysis to from OH ion.To calculat PH, suitable approximation is :a)[CO32-] = [HCO3]b)degree of ionization = degree of Hydrolysisc)both (A) and (B)d)neither A nore BCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Consider a solution of CH3COONH4 which is a salt of weak acid weak base. Theequilibrium involved in the solutions are :If we add these three reactions, then the net reaction isBoth CH3C00- and NH4 get hydrolysed independently and their hydrolysis depends on(i) their initial concentration(ii) the value of Kh which is Since both of the ions were produced from the same salt, their initial concentrations are same . Therefore unless untial the value of Kw/Kaand Kb is same, the degree of hydrolysis of ion cant be same.To explain why we assume that degree of hydrolysis of cation and anion is same, we need to now look at the third reaction i.e., combination of H+ and OH ions. It is obvious that this reaction happens only because one reaction produced H+ ion and the other produced OH ions. We can also note that this reaction causes both the hydrolysis reaction to occur more since their product ions are being consumed. Keep this thing in mind that the equilibrium which has smaller value of equilibrium conxtant is affected more by the common ion effect. For the same reason if for any reason a reaction is made to occur to a greater extent by the consumption of any one of the product ion, the reaction with the smaller value of equilibrium constant tends to get affected more.Therefore we conclude that firstly the hydrolysis of both the ions ocurs more in the presence of each other (due to consumption of the product ions) than in each other is absence. Secondly the hydrolysis of the ion which occurs to a lesser extent (due to smaller value of Kh) is affected more than the one whose Kh is greater. Hence we can see that the degree of hydrolysis of both the ions would be close to each other when they are getting hydrolysed in the presence of each other.Q.In a solution of NaHCO3 , the amphiprotic anion can under ionization to form H+ ion and hydrolysis to from OH ion.To calculat PH, suitable approximation is :a)[CO32-] = [HCO3]b)degree of ionization = degree of Hydrolysisc)both (A) and (B)d)neither A nore BCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Consider a solution of CH3COONH4 which is a salt of weak acid weak base. Theequilibrium involved in the solutions are :If we add these three reactions, then the net reaction isBoth CH3C00- and NH4 get hydrolysed independently and their hydrolysis depends on(i) their initial concentration(ii) the value of Kh which is Since both of the ions were produced from the same salt, their initial concentrations are same . Therefore unless untial the value of Kw/Kaand Kb is same, the degree of hydrolysis of ion cant be same.To explain why we assume that degree of hydrolysis of cation and anion is same, we need to now look at the third reaction i.e., combination of H+ and OH ions. It is obvious that this reaction happens only because one reaction produced H+ ion and the other produced OH ions. We can also note that this reaction causes both the hydrolysis reaction to occur more since their product ions are being consumed. Keep this thing in mind that the equilibrium which has smaller value of equilibrium conxtant is affected more by the common ion effect. For the same reason if for any reason a reaction is made to occur to a greater extent by the consumption of any one of the product ion, the reaction with the smaller value of equilibrium constant tends to get affected more.Therefore we conclude that firstly the hydrolysis of both the ions ocurs more in the presence of each other (due to consumption of the product ions) than in each other is absence. Secondly the hydrolysis of the ion which occurs to a lesser extent (due to smaller value of Kh) is affected more than the one whose Kh is greater. Hence we can see that the degree of hydrolysis of both the ions would be close to each other when they are getting hydrolysed in the presence of each other.Q.In a solution of NaHCO3 , the amphiprotic anion can under ionization to form H+ ion and hydrolysis to from OH ion.To calculat PH, suitable approximation is :a)[CO32-] = [HCO3]b)degree of ionization = degree of Hydrolysisc)both (A) and (B)d)neither A nore BCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Consider a solution of CH3COONH4 which is a salt of weak acid weak base. Theequilibrium involved in the solutions are :If we add these three reactions, then the net reaction isBoth CH3C00- and NH4 get hydrolysed independently and their hydrolysis depends on(i) their initial concentration(ii) the value of Kh which is Since both of the ions were produced from the same salt, their initial concentrations are same . Therefore unless untial the value of Kw/Kaand Kb is same, the degree of hydrolysis of ion cant be same.To explain why we assume that degree of hydrolysis of cation and anion is same, we need to now look at the third reaction i.e., combination of H+ and OH ions. It is obvious that this reaction happens only because one reaction produced H+ ion and the other produced OH ions. We can also note that this reaction causes both the hydrolysis reaction to occur more since their product ions are being consumed. Keep this thing in mind that the equilibrium which has smaller value of equilibrium conxtant is affected more by the common ion effect. For the same reason if for any reason a reaction is made to occur to a greater extent by the consumption of any one of the product ion, the reaction with the smaller value of equilibrium constant tends to get affected more.Therefore we conclude that firstly the hydrolysis of both the ions ocurs more in the presence of each other (due to consumption of the product ions) than in each other is absence. Secondly the hydrolysis of the ion which occurs to a lesser extent (due to smaller value of Kh) is affected more than the one whose Kh is greater. Hence we can see that the degree of hydrolysis of both the ions would be close to each other when they are getting hydrolysed in the presence of each other.Q.In a solution of NaHCO3 , the amphiprotic anion can under ionization to form H+ ion and hydrolysis to from OH ion.To calculat PH, suitable approximation is :a)[CO32-] = [HCO3]b)degree of ionization = degree of Hydrolysisc)both (A) and (B)d)neither A nore BCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























