IIT JAM Exam > IIT JAM Questions > Please give mechanism of Pinacol Pinacolone r...
Start Learning for Free
Please give mechanism of Pinacol Pinacolone rearrangement in case of cycling compounds.?
Most Upvoted Answer
Please give mechanism of Pinacol Pinacolone rearrangement in case of c...
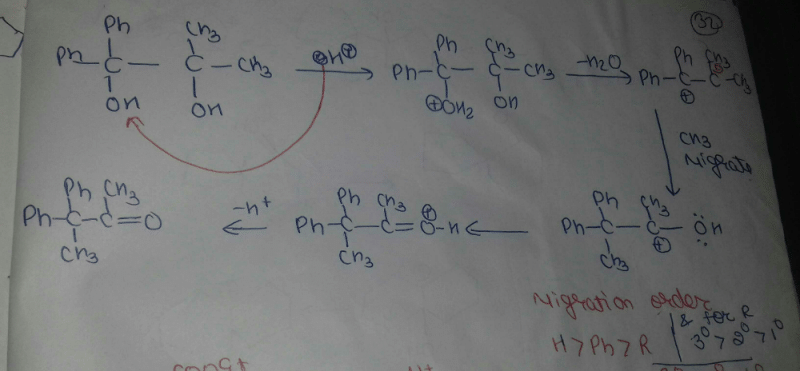
Community Answer
Please give mechanism of Pinacol Pinacolone rearrangement in case of c...
Pinacol Pinacolone Rearrangement Overview
The Pinacol Pinacolone rearrangement is a classic organic reaction involving the conversion of a pinacol into a ketone. This rearrangement is particularly interesting when considering cyclic compounds.
Mechanism Steps
1. Formation of the Pinacol
- The reaction begins with the formation of a pinacol from the corresponding carbonyl compound. This involves the nucleophilic attack of an alcohol on a carbonyl carbon, resulting in a 1,2-diol.
2. Protonation of the Alcohol
- In acidic conditions, one of the hydroxyl groups of the pinacol is protonated. This generates a good leaving group (water) and forms a carbocation at the adjacent carbon.
3. Carbocation Rearrangement
- The carbocation can undergo rearrangement. In case of cyclic compounds, this often leads to a more stable tertiary carbocation. The cyclic structure can stabilize the transition state due to ring strain release or through favorable orbital overlap.
4. Migration of the Group
- A group (typically a methyl or alkyl group) adjacent to the carbocation migrates to the positively charged carbon. This migration can occur in a concerted manner or via a stable carbocation intermediate.
5. Deprotonation
- Finally, the carbocation is deprotonated by a base (often the conjugate base of the acid used), resulting in the formation of a ketone, specifically pinacolone.
Significance of the Rearrangement
- The Pinacol Pinacolone rearrangement is significant in synthetic organic chemistry for constructing ketones from relatively simple precursors.
- It highlights the importance of carbocation stability, particularly in cyclic systems, where the structure can influence the reaction pathway.
This rearrangement showcases key concepts in organic reaction mechanisms, including carbocation stability, rearrangement, and the influence of cyclic structures.
The Pinacol Pinacolone rearrangement is a classic organic reaction involving the conversion of a pinacol into a ketone. This rearrangement is particularly interesting when considering cyclic compounds.
Mechanism Steps
1. Formation of the Pinacol
- The reaction begins with the formation of a pinacol from the corresponding carbonyl compound. This involves the nucleophilic attack of an alcohol on a carbonyl carbon, resulting in a 1,2-diol.
2. Protonation of the Alcohol
- In acidic conditions, one of the hydroxyl groups of the pinacol is protonated. This generates a good leaving group (water) and forms a carbocation at the adjacent carbon.
3. Carbocation Rearrangement
- The carbocation can undergo rearrangement. In case of cyclic compounds, this often leads to a more stable tertiary carbocation. The cyclic structure can stabilize the transition state due to ring strain release or through favorable orbital overlap.
4. Migration of the Group
- A group (typically a methyl or alkyl group) adjacent to the carbocation migrates to the positively charged carbon. This migration can occur in a concerted manner or via a stable carbocation intermediate.
5. Deprotonation
- Finally, the carbocation is deprotonated by a base (often the conjugate base of the acid used), resulting in the formation of a ketone, specifically pinacolone.
Significance of the Rearrangement
- The Pinacol Pinacolone rearrangement is significant in synthetic organic chemistry for constructing ketones from relatively simple precursors.
- It highlights the importance of carbocation stability, particularly in cyclic systems, where the structure can influence the reaction pathway.
This rearrangement showcases key concepts in organic reaction mechanisms, including carbocation stability, rearrangement, and the influence of cyclic structures.

|
Explore Courses for IIT JAM exam
|

|
Similar IIT JAM Doubts
Please give mechanism of Pinacol Pinacolone rearrangement in case of cycling compounds.?
Question Description
Please give mechanism of Pinacol Pinacolone rearrangement in case of cycling compounds.? for IIT JAM 2024 is part of IIT JAM preparation. The Question and answers have been prepared according to the IIT JAM exam syllabus. Information about Please give mechanism of Pinacol Pinacolone rearrangement in case of cycling compounds.? covers all topics & solutions for IIT JAM 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Please give mechanism of Pinacol Pinacolone rearrangement in case of cycling compounds.?.
Please give mechanism of Pinacol Pinacolone rearrangement in case of cycling compounds.? for IIT JAM 2024 is part of IIT JAM preparation. The Question and answers have been prepared according to the IIT JAM exam syllabus. Information about Please give mechanism of Pinacol Pinacolone rearrangement in case of cycling compounds.? covers all topics & solutions for IIT JAM 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Please give mechanism of Pinacol Pinacolone rearrangement in case of cycling compounds.?.
Solutions for Please give mechanism of Pinacol Pinacolone rearrangement in case of cycling compounds.? in English & in Hindi are available as part of our courses for IIT JAM.
Download more important topics, notes, lectures and mock test series for IIT JAM Exam by signing up for free.
Here you can find the meaning of Please give mechanism of Pinacol Pinacolone rearrangement in case of cycling compounds.? defined & explained in the simplest way possible. Besides giving the explanation of
Please give mechanism of Pinacol Pinacolone rearrangement in case of cycling compounds.?, a detailed solution for Please give mechanism of Pinacol Pinacolone rearrangement in case of cycling compounds.? has been provided alongside types of Please give mechanism of Pinacol Pinacolone rearrangement in case of cycling compounds.? theory, EduRev gives you an
ample number of questions to practice Please give mechanism of Pinacol Pinacolone rearrangement in case of cycling compounds.? tests, examples and also practice IIT JAM tests.

|
Explore Courses for IIT JAM exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















