JEE Exam > JEE Questions > Select the correctstatement (s)a)8 Cs+ions oc...
Start Learning for Free
Select the correct statement (s)
- a)8 Cs+ ions occupy the second nearest neighbour locations of a Cs+ ion
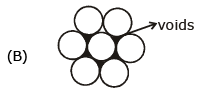
- b)Each sphere is surrounded by six voids in two dimensional hexagonal close packed layer
- c)If the radius of cations and amions are 0.3 Å and 0.4 Å then coordinate number of cation of the crystal is 6.
- d)In AgCl, the silver ion is displaced from its lattice position to an interstitial position such a defect is called a frenkel defect.
Correct answer is option 'B,D'. Can you explain this answer?
Verified Answer
Select the correctstatement (s)a)8 Cs+ions occupy the second nearest n...
(A) 6 Cs+ ion second nearest neighbour
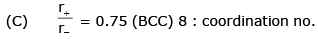
(D) True
(D) True
Most Upvoted Answer
Select the correctstatement (s)a)8 Cs+ions occupy the second nearest n...
Statement a) 8 Cs ions occupy the second nearest neighbour locations of a Cs ion
This statement is incorrect. In a crystal lattice, each ion is surrounded by its nearest neighbors, which are the ions closest to it. The second nearest neighbors are the ions that are further away. In a Cs ion lattice, the second nearest neighbor locations of a Cs ion would be occupied by other ions, not Cs ions themselves.
Statement b) Each sphere is surrounded by six voids in two-dimensional hexagonal close-packed layer
This statement is correct. In a two-dimensional hexagonal close-packed (HCP) layer, each sphere is surrounded by six voids. This is because the HCP structure consists of a repeating pattern of hexagonal layers, where each sphere is in contact with six other spheres. The voids are the spaces between these spheres, and each sphere is surrounded by six of these voids.
Statement c) If the radius of cations and anions are 0.3 and 0.4, then the coordination number of the cation of the crystal is 6
This statement is incorrect. The coordination number of an ion in a crystal refers to the number of ions that are in close proximity to it. It is determined by the radius ratio of the cations and anions. In general, if the radius ratio is less than a certain value, the coordination number is 4, and if it is greater than that value, the coordination number is 6. Therefore, the coordination number cannot be determined solely based on the given radii of the cations and anions.
Statement d) In AgCl, the silver ion is displaced from its lattice position to an interstitial position. Such a defect is called a Frenkel defect.
This statement is correct. In a Frenkel defect, an ion is displaced from its lattice position and occupies an interstitial position within the crystal lattice. In the case of AgCl, the silver ion (Ag+) is smaller than the chloride ion (Cl-) and can easily move into an interstitial position without disrupting the overall structure of the crystal lattice. This type of defect is commonly observed in ionic compounds with large differences in ion sizes.
This statement is incorrect. In a crystal lattice, each ion is surrounded by its nearest neighbors, which are the ions closest to it. The second nearest neighbors are the ions that are further away. In a Cs ion lattice, the second nearest neighbor locations of a Cs ion would be occupied by other ions, not Cs ions themselves.
Statement b) Each sphere is surrounded by six voids in two-dimensional hexagonal close-packed layer
This statement is correct. In a two-dimensional hexagonal close-packed (HCP) layer, each sphere is surrounded by six voids. This is because the HCP structure consists of a repeating pattern of hexagonal layers, where each sphere is in contact with six other spheres. The voids are the spaces between these spheres, and each sphere is surrounded by six of these voids.
Statement c) If the radius of cations and anions are 0.3 and 0.4, then the coordination number of the cation of the crystal is 6
This statement is incorrect. The coordination number of an ion in a crystal refers to the number of ions that are in close proximity to it. It is determined by the radius ratio of the cations and anions. In general, if the radius ratio is less than a certain value, the coordination number is 4, and if it is greater than that value, the coordination number is 6. Therefore, the coordination number cannot be determined solely based on the given radii of the cations and anions.
Statement d) In AgCl, the silver ion is displaced from its lattice position to an interstitial position. Such a defect is called a Frenkel defect.
This statement is correct. In a Frenkel defect, an ion is displaced from its lattice position and occupies an interstitial position within the crystal lattice. In the case of AgCl, the silver ion (Ag+) is smaller than the chloride ion (Cl-) and can easily move into an interstitial position without disrupting the overall structure of the crystal lattice. This type of defect is commonly observed in ionic compounds with large differences in ion sizes.

|
Explore Courses for JEE exam
|

|
Question Description
Select the correctstatement (s)a)8 Cs+ions occupy the second nearest neighbour locations of a Cs+ionb)Each sphere is surrounded by six voids in two dimensional hexagonal close packed layerc)If the radius of cations and amions are 0.3 and 0.4 then coordinate number of cation of the crystal is 6.d)In AgCl, the silver ion is displaced from its lattice position to an interstitial position such a defect is called a frenkel defect.Correct answer is option 'B,D'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Select the correctstatement (s)a)8 Cs+ions occupy the second nearest neighbour locations of a Cs+ionb)Each sphere is surrounded by six voids in two dimensional hexagonal close packed layerc)If the radius of cations and amions are 0.3 and 0.4 then coordinate number of cation of the crystal is 6.d)In AgCl, the silver ion is displaced from its lattice position to an interstitial position such a defect is called a frenkel defect.Correct answer is option 'B,D'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Select the correctstatement (s)a)8 Cs+ions occupy the second nearest neighbour locations of a Cs+ionb)Each sphere is surrounded by six voids in two dimensional hexagonal close packed layerc)If the radius of cations and amions are 0.3 and 0.4 then coordinate number of cation of the crystal is 6.d)In AgCl, the silver ion is displaced from its lattice position to an interstitial position such a defect is called a frenkel defect.Correct answer is option 'B,D'. Can you explain this answer?.
Select the correctstatement (s)a)8 Cs+ions occupy the second nearest neighbour locations of a Cs+ionb)Each sphere is surrounded by six voids in two dimensional hexagonal close packed layerc)If the radius of cations and amions are 0.3 and 0.4 then coordinate number of cation of the crystal is 6.d)In AgCl, the silver ion is displaced from its lattice position to an interstitial position such a defect is called a frenkel defect.Correct answer is option 'B,D'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Select the correctstatement (s)a)8 Cs+ions occupy the second nearest neighbour locations of a Cs+ionb)Each sphere is surrounded by six voids in two dimensional hexagonal close packed layerc)If the radius of cations and amions are 0.3 and 0.4 then coordinate number of cation of the crystal is 6.d)In AgCl, the silver ion is displaced from its lattice position to an interstitial position such a defect is called a frenkel defect.Correct answer is option 'B,D'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Select the correctstatement (s)a)8 Cs+ions occupy the second nearest neighbour locations of a Cs+ionb)Each sphere is surrounded by six voids in two dimensional hexagonal close packed layerc)If the radius of cations and amions are 0.3 and 0.4 then coordinate number of cation of the crystal is 6.d)In AgCl, the silver ion is displaced from its lattice position to an interstitial position such a defect is called a frenkel defect.Correct answer is option 'B,D'. Can you explain this answer?.
Solutions for Select the correctstatement (s)a)8 Cs+ions occupy the second nearest neighbour locations of a Cs+ionb)Each sphere is surrounded by six voids in two dimensional hexagonal close packed layerc)If the radius of cations and amions are 0.3 and 0.4 then coordinate number of cation of the crystal is 6.d)In AgCl, the silver ion is displaced from its lattice position to an interstitial position such a defect is called a frenkel defect.Correct answer is option 'B,D'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Select the correctstatement (s)a)8 Cs+ions occupy the second nearest neighbour locations of a Cs+ionb)Each sphere is surrounded by six voids in two dimensional hexagonal close packed layerc)If the radius of cations and amions are 0.3 and 0.4 then coordinate number of cation of the crystal is 6.d)In AgCl, the silver ion is displaced from its lattice position to an interstitial position such a defect is called a frenkel defect.Correct answer is option 'B,D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Select the correctstatement (s)a)8 Cs+ions occupy the second nearest neighbour locations of a Cs+ionb)Each sphere is surrounded by six voids in two dimensional hexagonal close packed layerc)If the radius of cations and amions are 0.3 and 0.4 then coordinate number of cation of the crystal is 6.d)In AgCl, the silver ion is displaced from its lattice position to an interstitial position such a defect is called a frenkel defect.Correct answer is option 'B,D'. Can you explain this answer?, a detailed solution for Select the correctstatement (s)a)8 Cs+ions occupy the second nearest neighbour locations of a Cs+ionb)Each sphere is surrounded by six voids in two dimensional hexagonal close packed layerc)If the radius of cations and amions are 0.3 and 0.4 then coordinate number of cation of the crystal is 6.d)In AgCl, the silver ion is displaced from its lattice position to an interstitial position such a defect is called a frenkel defect.Correct answer is option 'B,D'. Can you explain this answer? has been provided alongside types of Select the correctstatement (s)a)8 Cs+ions occupy the second nearest neighbour locations of a Cs+ionb)Each sphere is surrounded by six voids in two dimensional hexagonal close packed layerc)If the radius of cations and amions are 0.3 and 0.4 then coordinate number of cation of the crystal is 6.d)In AgCl, the silver ion is displaced from its lattice position to an interstitial position such a defect is called a frenkel defect.Correct answer is option 'B,D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Select the correctstatement (s)a)8 Cs+ions occupy the second nearest neighbour locations of a Cs+ionb)Each sphere is surrounded by six voids in two dimensional hexagonal close packed layerc)If the radius of cations and amions are 0.3 and 0.4 then coordinate number of cation of the crystal is 6.d)In AgCl, the silver ion is displaced from its lattice position to an interstitial position such a defect is called a frenkel defect.Correct answer is option 'B,D'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















