Class 12 Exam > Class 12 Questions > Finkelstein reaction?
Start Learning for Free
Finkelstein reaction?
Most Upvoted Answer
Finkelstein reaction?
**Finkelstein Reaction: An Introduction**
The Finkelstein reaction is a well-known organic reaction that involves the conversion of an alkyl halide to another alkyl halide with a different halogen atom. It is named after the German chemist Hans Finkelstein, who first discovered and studied this reaction in the early 20th century. The Finkelstein reaction is commonly used in organic synthesis and finds applications in various fields, including pharmaceuticals, agrochemicals, and materials science.
**Mechanism of the Finkelstein Reaction**
The Finkelstein reaction is typically carried out by treating an alkyl halide with a solution of a different halide salt in an aprotic polar solvent. The reaction proceeds via a nucleophilic substitution mechanism, involving the exchange of the halogen atom in the alkyl halide with the halide ion from the salt.
The general mechanism of the Finkelstein reaction can be summarized as follows:
1. Nucleophilic attack: The halide ion (X-) from the salt acts as a nucleophile and attacks the carbon atom of the alkyl halide, resulting in the formation of a transition state.
2. Formation of a new bond: The carbon-halogen bond in the alkyl halide breaks, and a new carbon-halogen bond is formed with the halide ion. This step leads to the displacement of the original halogen atom with the new halogen atom.
3. Ionization: The halide ion from the salt becomes the leaving group, and it dissociates from the reaction mixture.
4. Solvent role: The aprotic polar solvent plays a crucial role in the Finkelstein reaction by solvating the ions, stabilizing the transition state, and facilitating the reaction.
**Factors Affecting the Finkelstein Reaction**
Several factors influence the rate and efficiency of the Finkelstein reaction. Some of the key factors include:
- Reactant structure: The reactivity of alkyl halides in the Finkelstein reaction depends on their structure, with primary alkyl halides being more reactive than secondary or tertiary alkyl halides.
- Halide strength: The relative strength of the halides involved in the reaction can also impact the reaction rate. Generally, iodide ions are more nucleophilic and better leaving groups compared to chloride, bromide, or fluoride ions.
- Solvent choice: The choice of aprotic polar solvent is crucial in the Finkelstein reaction. Common solvents include acetone, acetonitrile, and dimethylformamide (DMF), which provide the necessary solvation and stabilization of the reaction intermediates.
**Applications of the Finkelstein Reaction**
The Finkelstein reaction has numerous applications in organic synthesis. Some notable applications include:
- Synthesis of pharmaceuticals: The Finkelstein reaction is often used to introduce specific halogen atoms into drug molecules, altering their properties and enhancing their pharmaceutical activity.
- Preparation of organohalides: The reaction is frequently employed to synthesize a wide range of organohalides, which serve as important intermediates in various organic transformations.
- Radiochemistry: The Finkelstein reaction is utilized in radiochemistry to prepare radioactive alkyl halides for labeling organic compounds with radioactive isotopes, enabling the study of biochemical processes.
- Materials science: The Finkelstein reaction plays a role in the synthesis of various materials, such as polymers and liquid crystals,
The Finkelstein reaction is a well-known organic reaction that involves the conversion of an alkyl halide to another alkyl halide with a different halogen atom. It is named after the German chemist Hans Finkelstein, who first discovered and studied this reaction in the early 20th century. The Finkelstein reaction is commonly used in organic synthesis and finds applications in various fields, including pharmaceuticals, agrochemicals, and materials science.
**Mechanism of the Finkelstein Reaction**
The Finkelstein reaction is typically carried out by treating an alkyl halide with a solution of a different halide salt in an aprotic polar solvent. The reaction proceeds via a nucleophilic substitution mechanism, involving the exchange of the halogen atom in the alkyl halide with the halide ion from the salt.
The general mechanism of the Finkelstein reaction can be summarized as follows:
1. Nucleophilic attack: The halide ion (X-) from the salt acts as a nucleophile and attacks the carbon atom of the alkyl halide, resulting in the formation of a transition state.
2. Formation of a new bond: The carbon-halogen bond in the alkyl halide breaks, and a new carbon-halogen bond is formed with the halide ion. This step leads to the displacement of the original halogen atom with the new halogen atom.
3. Ionization: The halide ion from the salt becomes the leaving group, and it dissociates from the reaction mixture.
4. Solvent role: The aprotic polar solvent plays a crucial role in the Finkelstein reaction by solvating the ions, stabilizing the transition state, and facilitating the reaction.
**Factors Affecting the Finkelstein Reaction**
Several factors influence the rate and efficiency of the Finkelstein reaction. Some of the key factors include:
- Reactant structure: The reactivity of alkyl halides in the Finkelstein reaction depends on their structure, with primary alkyl halides being more reactive than secondary or tertiary alkyl halides.
- Halide strength: The relative strength of the halides involved in the reaction can also impact the reaction rate. Generally, iodide ions are more nucleophilic and better leaving groups compared to chloride, bromide, or fluoride ions.
- Solvent choice: The choice of aprotic polar solvent is crucial in the Finkelstein reaction. Common solvents include acetone, acetonitrile, and dimethylformamide (DMF), which provide the necessary solvation and stabilization of the reaction intermediates.
**Applications of the Finkelstein Reaction**
The Finkelstein reaction has numerous applications in organic synthesis. Some notable applications include:
- Synthesis of pharmaceuticals: The Finkelstein reaction is often used to introduce specific halogen atoms into drug molecules, altering their properties and enhancing their pharmaceutical activity.
- Preparation of organohalides: The reaction is frequently employed to synthesize a wide range of organohalides, which serve as important intermediates in various organic transformations.
- Radiochemistry: The Finkelstein reaction is utilized in radiochemistry to prepare radioactive alkyl halides for labeling organic compounds with radioactive isotopes, enabling the study of biochemical processes.
- Materials science: The Finkelstein reaction plays a role in the synthesis of various materials, such as polymers and liquid crystals,
Community Answer
Finkelstein reaction?
The Finkelstein reaction is an organic reaction where an alkyl halide is converted into another alkyl halide by reacting with a metal halide salt.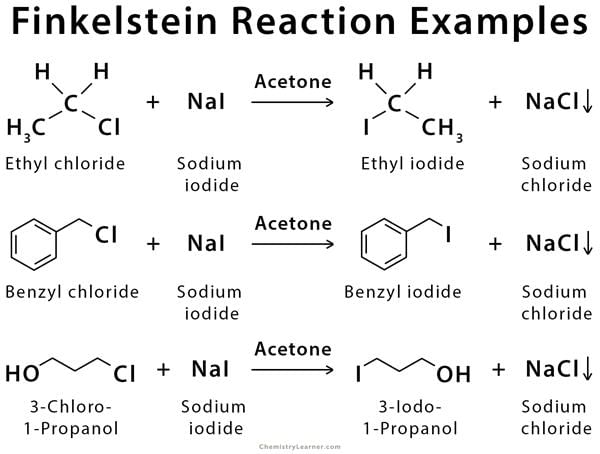


|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Finkelstein reaction?
Question Description
Finkelstein reaction? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Finkelstein reaction? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Finkelstein reaction?.
Finkelstein reaction? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Finkelstein reaction? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Finkelstein reaction?.
Solutions for Finkelstein reaction? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Finkelstein reaction? defined & explained in the simplest way possible. Besides giving the explanation of
Finkelstein reaction?, a detailed solution for Finkelstein reaction? has been provided alongside types of Finkelstein reaction? theory, EduRev gives you an
ample number of questions to practice Finkelstein reaction? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



















