JEE Exam > JEE Questions > Which of the following statements is(are) fal...
Start Learning for Free
Which of the following statements is(are) false ?
- a)XeO3 is a colourless explosive solid and has a pyramidal molecular structure.
- b)XeF2 is not a better oxidising agent.
- c)Xe, Kr and Ne all form clatharate compounds.
- d)Fluorine in dilute solution of sodium hydroxide disproportionates in fluoride and hypofluorite.
Correct answer is option 'B,C,D'. Can you explain this answer?
Verified Answer
Which of the following statements is(are) false ?a)XeO3 is a colourles...
(A) Statement is correct 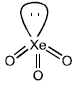
(B) Statement is false.
2e- + 2H+ + XeF2 → Xe + 2HF
SRP = +2.64V.
(C) Statement is false as Ne atoms being smaller do not trapp in the cavities formed by water molecules (ice) and
thus does not form clatharte compounds. Xe and Kr being bigger form clathrate compounds.
(D) Statement is false according to the following reaction : 2F2 + 2NaOH → OF2(g) + 2NaF + H2O.
2e- + 2H+ + XeF2 → Xe + 2HF
SRP = +2.64V.
(C) Statement is false as Ne atoms being smaller do not trapp in the cavities formed by water molecules (ice) and
thus does not form clatharte compounds. Xe and Kr being bigger form clathrate compounds.
(D) Statement is false according to the following reaction : 2F2 + 2NaOH → OF2(g) + 2NaF + H2O.
Most Upvoted Answer
Which of the following statements is(are) false ?a)XeO3 is a colourles...
False Statements:
- XeF2 is not a better oxidizing agent.
- Xe, Kr and Ne all form clatharate compounds.
- Fluorine in dilute solution of sodium hydroxide disproportionates in fluoride and hypofluorite.
Explanation:
XeO3:
- XeO3 is a pale-yellow explosive solid and has a pyramidal molecular structure.
- It is formed by the reaction of xenon hexafluoride with water and has a trigonal pyramidal shape due to the presence of one lone pair of electrons on the central xenon atom.
- The Xe-O bonds are polar with oxygen being more electronegative than xenon.
XeF2:
- XeF2 is a colorless and odorless gas and is a better oxidizing agent than Xe.
- It is a powerful fluorinating agent and can react with a variety of compounds including metals, nonmetals, and noble gases.
- It has a linear molecular structure due to the presence of two lone pairs of electrons on the central xenon atom.
Clatharate Compounds:
- Clatharate compounds are inclusion compounds in which small guest molecules are trapped within the cavities of a host lattice.
- Xenon, krypton, and neon can form clatharate compounds with water, alcohols, and some other polar molecules.
- The guest molecules are trapped in the host lattice by weak intermolecular forces such as van der Waals forces.
Fluorine Disproportionation:
- Disproportionation is a redox reaction in which an element is simultaneously oxidized and reduced.
- When fluorine is added to a dilute solution of sodium hydroxide, it disproportionates into fluoride and hypofluorite ions.
- The overall reaction can be represented as 3F2 + 6NaOH → 5NaF + NaOCl + 3H2O.
Conclusion:
XeO3 is a pale-yellow explosive solid and has a pyramidal molecular structure. XeF2 is a colorless and odorless gas and is a better oxidizing agent than Xe. Xenon, krypton, and neon can form clatharate compounds with water, alcohols, and some other polar molecules. When fluorine is added to a dilute solution of sodium hydroxide, it disproportionates into fluoride and hypofluorite ions.
- XeF2 is not a better oxidizing agent.
- Xe, Kr and Ne all form clatharate compounds.
- Fluorine in dilute solution of sodium hydroxide disproportionates in fluoride and hypofluorite.
Explanation:
XeO3:
- XeO3 is a pale-yellow explosive solid and has a pyramidal molecular structure.
- It is formed by the reaction of xenon hexafluoride with water and has a trigonal pyramidal shape due to the presence of one lone pair of electrons on the central xenon atom.
- The Xe-O bonds are polar with oxygen being more electronegative than xenon.
XeF2:
- XeF2 is a colorless and odorless gas and is a better oxidizing agent than Xe.
- It is a powerful fluorinating agent and can react with a variety of compounds including metals, nonmetals, and noble gases.
- It has a linear molecular structure due to the presence of two lone pairs of electrons on the central xenon atom.
Clatharate Compounds:
- Clatharate compounds are inclusion compounds in which small guest molecules are trapped within the cavities of a host lattice.
- Xenon, krypton, and neon can form clatharate compounds with water, alcohols, and some other polar molecules.
- The guest molecules are trapped in the host lattice by weak intermolecular forces such as van der Waals forces.
Fluorine Disproportionation:
- Disproportionation is a redox reaction in which an element is simultaneously oxidized and reduced.
- When fluorine is added to a dilute solution of sodium hydroxide, it disproportionates into fluoride and hypofluorite ions.
- The overall reaction can be represented as 3F2 + 6NaOH → 5NaF + NaOCl + 3H2O.
Conclusion:
XeO3 is a pale-yellow explosive solid and has a pyramidal molecular structure. XeF2 is a colorless and odorless gas and is a better oxidizing agent than Xe. Xenon, krypton, and neon can form clatharate compounds with water, alcohols, and some other polar molecules. When fluorine is added to a dilute solution of sodium hydroxide, it disproportionates into fluoride and hypofluorite ions.

|
Explore Courses for JEE exam
|

|
Question Description
Which of the following statements is(are) false ?a)XeO3 is a colourless explosive solid and has a pyramidal molecular structure.b)XeF2 is not a better oxidising agent.c)Xe, Kr and Ne all form clatharate compounds.d)Fluorine in dilute solution of sodium hydroxide disproportionates in fluoride and hypofluorite.Correct answer is option 'B,C,D'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Which of the following statements is(are) false ?a)XeO3 is a colourless explosive solid and has a pyramidal molecular structure.b)XeF2 is not a better oxidising agent.c)Xe, Kr and Ne all form clatharate compounds.d)Fluorine in dilute solution of sodium hydroxide disproportionates in fluoride and hypofluorite.Correct answer is option 'B,C,D'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following statements is(are) false ?a)XeO3 is a colourless explosive solid and has a pyramidal molecular structure.b)XeF2 is not a better oxidising agent.c)Xe, Kr and Ne all form clatharate compounds.d)Fluorine in dilute solution of sodium hydroxide disproportionates in fluoride and hypofluorite.Correct answer is option 'B,C,D'. Can you explain this answer?.
Which of the following statements is(are) false ?a)XeO3 is a colourless explosive solid and has a pyramidal molecular structure.b)XeF2 is not a better oxidising agent.c)Xe, Kr and Ne all form clatharate compounds.d)Fluorine in dilute solution of sodium hydroxide disproportionates in fluoride and hypofluorite.Correct answer is option 'B,C,D'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Which of the following statements is(are) false ?a)XeO3 is a colourless explosive solid and has a pyramidal molecular structure.b)XeF2 is not a better oxidising agent.c)Xe, Kr and Ne all form clatharate compounds.d)Fluorine in dilute solution of sodium hydroxide disproportionates in fluoride and hypofluorite.Correct answer is option 'B,C,D'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following statements is(are) false ?a)XeO3 is a colourless explosive solid and has a pyramidal molecular structure.b)XeF2 is not a better oxidising agent.c)Xe, Kr and Ne all form clatharate compounds.d)Fluorine in dilute solution of sodium hydroxide disproportionates in fluoride and hypofluorite.Correct answer is option 'B,C,D'. Can you explain this answer?.
Solutions for Which of the following statements is(are) false ?a)XeO3 is a colourless explosive solid and has a pyramidal molecular structure.b)XeF2 is not a better oxidising agent.c)Xe, Kr and Ne all form clatharate compounds.d)Fluorine in dilute solution of sodium hydroxide disproportionates in fluoride and hypofluorite.Correct answer is option 'B,C,D'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Which of the following statements is(are) false ?a)XeO3 is a colourless explosive solid and has a pyramidal molecular structure.b)XeF2 is not a better oxidising agent.c)Xe, Kr and Ne all form clatharate compounds.d)Fluorine in dilute solution of sodium hydroxide disproportionates in fluoride and hypofluorite.Correct answer is option 'B,C,D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following statements is(are) false ?a)XeO3 is a colourless explosive solid and has a pyramidal molecular structure.b)XeF2 is not a better oxidising agent.c)Xe, Kr and Ne all form clatharate compounds.d)Fluorine in dilute solution of sodium hydroxide disproportionates in fluoride and hypofluorite.Correct answer is option 'B,C,D'. Can you explain this answer?, a detailed solution for Which of the following statements is(are) false ?a)XeO3 is a colourless explosive solid and has a pyramidal molecular structure.b)XeF2 is not a better oxidising agent.c)Xe, Kr and Ne all form clatharate compounds.d)Fluorine in dilute solution of sodium hydroxide disproportionates in fluoride and hypofluorite.Correct answer is option 'B,C,D'. Can you explain this answer? has been provided alongside types of Which of the following statements is(are) false ?a)XeO3 is a colourless explosive solid and has a pyramidal molecular structure.b)XeF2 is not a better oxidising agent.c)Xe, Kr and Ne all form clatharate compounds.d)Fluorine in dilute solution of sodium hydroxide disproportionates in fluoride and hypofluorite.Correct answer is option 'B,C,D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following statements is(are) false ?a)XeO3 is a colourless explosive solid and has a pyramidal molecular structure.b)XeF2 is not a better oxidising agent.c)Xe, Kr and Ne all form clatharate compounds.d)Fluorine in dilute solution of sodium hydroxide disproportionates in fluoride and hypofluorite.Correct answer is option 'B,C,D'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















