Mechanical Engineering Exam > Mechanical Engineering Questions > Which one of the following have a highest the...
Start Learning for Free
Which one of the following have a highest thermal conductivity?
- a)Boiling water
- b)Steam
- c)Solid ice
- d)Rainwa
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
Which one of the following have a highest thermal conductivity?a)Boili...
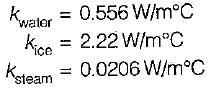
Most Upvoted Answer
Which one of the following have a highest thermal conductivity?a)Boili...
Understanding Thermal Conductivity
Thermal conductivity is a measure of a material's ability to conduct heat. It varies significantly across different states of matter (solid, liquid, gas).
Factors Affecting Thermal Conductivity
- State of Matter: Solids generally have higher thermal conductivity than liquids and gases due to closely packed molecules.
- Molecular Structure: The arrangement and bonding of atoms affect how easily heat can be transferred.
Comparison of Options
- Boiling Water: While water can conduct heat, its thermal conductivity is lower than that of solids. Its value is about 0.6 W/m·K.
- Steam: As a gas, steam has a lower thermal conductivity compared to liquids and solids. The thermal conductivity of steam is around 0.02 W/m·K.
- Solid Ice: Ice, being a solid, has a higher thermal conductivity than water in liquid form. The thermal conductivity of ice is approximately 2.2 W/m·K.
- Rainwater: Similar to boiling water, rainwater has low thermal conductivity, comparable to that of boiling water.
Conclusion
Based on the comparison, solid ice (option C) possesses the highest thermal conductivity among the listed options. This is due to its solid state, which allows for efficient heat transfer through tightly packed molecular structures. In contrast, the other options (boiling water, steam, and rainwater) are either in liquid or gaseous states, resulting in lower thermal conductivity.
Thus, the correct answer is indeed option C: Solid Ice.
Thermal conductivity is a measure of a material's ability to conduct heat. It varies significantly across different states of matter (solid, liquid, gas).
Factors Affecting Thermal Conductivity
- State of Matter: Solids generally have higher thermal conductivity than liquids and gases due to closely packed molecules.
- Molecular Structure: The arrangement and bonding of atoms affect how easily heat can be transferred.
Comparison of Options
- Boiling Water: While water can conduct heat, its thermal conductivity is lower than that of solids. Its value is about 0.6 W/m·K.
- Steam: As a gas, steam has a lower thermal conductivity compared to liquids and solids. The thermal conductivity of steam is around 0.02 W/m·K.
- Solid Ice: Ice, being a solid, has a higher thermal conductivity than water in liquid form. The thermal conductivity of ice is approximately 2.2 W/m·K.
- Rainwater: Similar to boiling water, rainwater has low thermal conductivity, comparable to that of boiling water.
Conclusion
Based on the comparison, solid ice (option C) possesses the highest thermal conductivity among the listed options. This is due to its solid state, which allows for efficient heat transfer through tightly packed molecular structures. In contrast, the other options (boiling water, steam, and rainwater) are either in liquid or gaseous states, resulting in lower thermal conductivity.
Thus, the correct answer is indeed option C: Solid Ice.

|
Explore Courses for Mechanical Engineering exam
|

|
Question Description
Which one of the following have a highest thermal conductivity?a)Boiling waterb)Steamc)Solid iced)RainwaCorrect answer is option 'C'. Can you explain this answer? for Mechanical Engineering 2025 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about Which one of the following have a highest thermal conductivity?a)Boiling waterb)Steamc)Solid iced)RainwaCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which one of the following have a highest thermal conductivity?a)Boiling waterb)Steamc)Solid iced)RainwaCorrect answer is option 'C'. Can you explain this answer?.
Which one of the following have a highest thermal conductivity?a)Boiling waterb)Steamc)Solid iced)RainwaCorrect answer is option 'C'. Can you explain this answer? for Mechanical Engineering 2025 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about Which one of the following have a highest thermal conductivity?a)Boiling waterb)Steamc)Solid iced)RainwaCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which one of the following have a highest thermal conductivity?a)Boiling waterb)Steamc)Solid iced)RainwaCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Which one of the following have a highest thermal conductivity?a)Boiling waterb)Steamc)Solid iced)RainwaCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Mechanical Engineering.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
Here you can find the meaning of Which one of the following have a highest thermal conductivity?a)Boiling waterb)Steamc)Solid iced)RainwaCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which one of the following have a highest thermal conductivity?a)Boiling waterb)Steamc)Solid iced)RainwaCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Which one of the following have a highest thermal conductivity?a)Boiling waterb)Steamc)Solid iced)RainwaCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Which one of the following have a highest thermal conductivity?a)Boiling waterb)Steamc)Solid iced)RainwaCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which one of the following have a highest thermal conductivity?a)Boiling waterb)Steamc)Solid iced)RainwaCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Mechanical Engineering tests.

|
Explore Courses for Mechanical Engineering exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























