Mechanical Engineering Exam > Mechanical Engineering Questions > When a real gas undergoes Joule-thomson expan...
Start Learning for Free
When a real gas undergoes Joule-thomson expansion the temperature
- a)may remain constant
- b)always increases
- c)may increase or decrease
- d)always decreases
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
When a real gas undergoes Joule-thomson expansion the temperaturea)may...
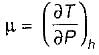
If μ is positive ⇒ temperature decreases during throttling
If μ is negative ⇒ Temperature increases during throttling.
Most Upvoted Answer
When a real gas undergoes Joule-thomson expansion the temperaturea)may...
Joule-Thomson Expansion of Real Gases
Joule-Thomson expansion, also known as the Joule-Thomson effect, is a process in which a real gas expands through a valve or a porous plug, resulting in a change in temperature. The Joule-Thomson coefficient is a measure of the temperature change that occurs during this expansion. The coefficient can be positive, negative, or zero, indicating whether the temperature increases, decreases, or remains constant during the expansion.
Explanation:
The correct answer to the given question is option 'C' - the temperature may increase or decrease during Joule-Thomson expansion of a real gas. This means that the temperature change is not fixed and can vary depending on the conditions.
Reasoning:
The temperature change during Joule-Thomson expansion is determined by the intermolecular forces and the nature of the gas molecules. Real gases deviate from ideal behavior at high pressures and low temperatures due to the presence of intermolecular forces. These forces can either enhance or diminish the temperature change during expansion.
Factors affecting temperature change:
1. Nature of the gas: Different gases have different intermolecular forces and molecular structures, which can affect the temperature change during expansion. For example, gases with weak intermolecular forces, such as noble gases, tend to exhibit a small temperature change during Joule-Thomson expansion.
2. Initial conditions: The pressure and temperature conditions of the gas before expansion also play a role in determining the temperature change. If the gas is initially at a high pressure and low temperature, the temperature is more likely to decrease during expansion. Conversely, if the gas is initially at a low pressure and high temperature, the temperature is more likely to increase during expansion.
3. Joule-Thomson coefficient: The coefficient itself can be positive or negative, indicating whether the temperature increases or decreases during expansion. The coefficient depends on the specific gas and its conditions.
Conclusion:
In summary, the temperature change during Joule-Thomson expansion of a real gas can vary depending on factors such as the nature of the gas, initial conditions, and the Joule-Thomson coefficient. Therefore, option 'C' - the temperature may increase or decrease - is the correct answer.
Joule-Thomson expansion, also known as the Joule-Thomson effect, is a process in which a real gas expands through a valve or a porous plug, resulting in a change in temperature. The Joule-Thomson coefficient is a measure of the temperature change that occurs during this expansion. The coefficient can be positive, negative, or zero, indicating whether the temperature increases, decreases, or remains constant during the expansion.
Explanation:
The correct answer to the given question is option 'C' - the temperature may increase or decrease during Joule-Thomson expansion of a real gas. This means that the temperature change is not fixed and can vary depending on the conditions.
Reasoning:
The temperature change during Joule-Thomson expansion is determined by the intermolecular forces and the nature of the gas molecules. Real gases deviate from ideal behavior at high pressures and low temperatures due to the presence of intermolecular forces. These forces can either enhance or diminish the temperature change during expansion.
Factors affecting temperature change:
1. Nature of the gas: Different gases have different intermolecular forces and molecular structures, which can affect the temperature change during expansion. For example, gases with weak intermolecular forces, such as noble gases, tend to exhibit a small temperature change during Joule-Thomson expansion.
2. Initial conditions: The pressure and temperature conditions of the gas before expansion also play a role in determining the temperature change. If the gas is initially at a high pressure and low temperature, the temperature is more likely to decrease during expansion. Conversely, if the gas is initially at a low pressure and high temperature, the temperature is more likely to increase during expansion.
3. Joule-Thomson coefficient: The coefficient itself can be positive or negative, indicating whether the temperature increases or decreases during expansion. The coefficient depends on the specific gas and its conditions.
Conclusion:
In summary, the temperature change during Joule-Thomson expansion of a real gas can vary depending on factors such as the nature of the gas, initial conditions, and the Joule-Thomson coefficient. Therefore, option 'C' - the temperature may increase or decrease - is the correct answer.

|
Explore Courses for Mechanical Engineering exam
|

|
Question Description
When a real gas undergoes Joule-thomson expansion the temperaturea)may remain constantb)always increasesc)may increase or decreased)always decreasesCorrect answer is option 'C'. Can you explain this answer? for Mechanical Engineering 2025 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about When a real gas undergoes Joule-thomson expansion the temperaturea)may remain constantb)always increasesc)may increase or decreased)always decreasesCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When a real gas undergoes Joule-thomson expansion the temperaturea)may remain constantb)always increasesc)may increase or decreased)always decreasesCorrect answer is option 'C'. Can you explain this answer?.
When a real gas undergoes Joule-thomson expansion the temperaturea)may remain constantb)always increasesc)may increase or decreased)always decreasesCorrect answer is option 'C'. Can you explain this answer? for Mechanical Engineering 2025 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about When a real gas undergoes Joule-thomson expansion the temperaturea)may remain constantb)always increasesc)may increase or decreased)always decreasesCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When a real gas undergoes Joule-thomson expansion the temperaturea)may remain constantb)always increasesc)may increase or decreased)always decreasesCorrect answer is option 'C'. Can you explain this answer?.
Solutions for When a real gas undergoes Joule-thomson expansion the temperaturea)may remain constantb)always increasesc)may increase or decreased)always decreasesCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Mechanical Engineering.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
Here you can find the meaning of When a real gas undergoes Joule-thomson expansion the temperaturea)may remain constantb)always increasesc)may increase or decreased)always decreasesCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
When a real gas undergoes Joule-thomson expansion the temperaturea)may remain constantb)always increasesc)may increase or decreased)always decreasesCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for When a real gas undergoes Joule-thomson expansion the temperaturea)may remain constantb)always increasesc)may increase or decreased)always decreasesCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of When a real gas undergoes Joule-thomson expansion the temperaturea)may remain constantb)always increasesc)may increase or decreased)always decreasesCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice When a real gas undergoes Joule-thomson expansion the temperaturea)may remain constantb)always increasesc)may increase or decreased)always decreasesCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Mechanical Engineering tests.

|
Explore Courses for Mechanical Engineering exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























