Mechanical Engineering Exam > Mechanical Engineering Questions > In a electrochemical machining ( E C M ) oper...
Start Learning for Free
In a electrochemical machining ( E C M ) operation, a square hole of dimensions 5 mm × mm is drilled in a block of copper. The current used is 5000 A. Atomic weight of copper is 63 and valence of dissolution is 1. Faradays constant is 96500 coulomb. The material removal rate (g/s) is
[2008]
- a)0.326
- b)3.26
- c)3.15 × 103
- d)3.15 × 105
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
In a electrochemical machining ( E C M ) operation, a square hole of d...
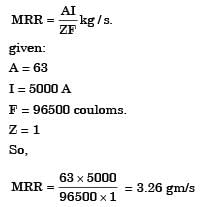
Most Upvoted Answer
In a electrochemical machining ( E C M ) operation, a square hole of d...
X 5 mm needs to be machined on a metal workpiece. The workpiece is placed in a tank filled with electrolyte solution and a tool electrode is also placed in the tank. An electrical potential is applied between the workpiece and the tool electrode to initiate the electrochemical reaction.
The electrolyte solution reacts with the metal workpiece, causing the metal ions to dissolve and form a slurry. The slurry is then flushed away from the workpiece, leaving the desired shape.
To machine a square hole of dimensions 5 mm x 5 mm, the tool electrode is designed to have a square shape with the same dimensions. The tool electrode is positioned directly above the workpiece and slowly lowered into the electrolyte solution.
The electrical potential is then applied and the electrochemical reaction begins, slowly dissolving the metal workpiece and creating the square hole. The tool electrode is moved around the workpiece to ensure that the entire hole is machined to the correct dimensions.
After the machining is complete, the workpiece is removed from the tank and cleaned to remove any remaining slurry. The resulting square hole is precise and accurate, with a smooth surface finish.
The electrolyte solution reacts with the metal workpiece, causing the metal ions to dissolve and form a slurry. The slurry is then flushed away from the workpiece, leaving the desired shape.
To machine a square hole of dimensions 5 mm x 5 mm, the tool electrode is designed to have a square shape with the same dimensions. The tool electrode is positioned directly above the workpiece and slowly lowered into the electrolyte solution.
The electrical potential is then applied and the electrochemical reaction begins, slowly dissolving the metal workpiece and creating the square hole. The tool electrode is moved around the workpiece to ensure that the entire hole is machined to the correct dimensions.
After the machining is complete, the workpiece is removed from the tank and cleaned to remove any remaining slurry. The resulting square hole is precise and accurate, with a smooth surface finish.
Attention Mechanical Engineering Students!
To make sure you are not studying endlessly, EduRev has designed Mechanical Engineering study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Mechanical Engineering.

|
Explore Courses for Mechanical Engineering exam
|

|
Similar Mechanical Engineering Doubts
In a electrochemical machining ( E C M ) operation, a square hole of dimensions 5 mm × mm is drilled in a block of copper. The current used is 5000 A. Atomic weight of copper is 63 and valence of dissolution is 1. Faradays constant is 96500 coulomb. The material removal rate (g/s) is[2008]a)0.326b)3.26c)3.15 × 103d)3.15 × 105Correct answer is option 'B'. Can you explain this answer?
Question Description
In a electrochemical machining ( E C M ) operation, a square hole of dimensions 5 mm × mm is drilled in a block of copper. The current used is 5000 A. Atomic weight of copper is 63 and valence of dissolution is 1. Faradays constant is 96500 coulomb. The material removal rate (g/s) is[2008]a)0.326b)3.26c)3.15 × 103d)3.15 × 105Correct answer is option 'B'. Can you explain this answer? for Mechanical Engineering 2024 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about In a electrochemical machining ( E C M ) operation, a square hole of dimensions 5 mm × mm is drilled in a block of copper. The current used is 5000 A. Atomic weight of copper is 63 and valence of dissolution is 1. Faradays constant is 96500 coulomb. The material removal rate (g/s) is[2008]a)0.326b)3.26c)3.15 × 103d)3.15 × 105Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In a electrochemical machining ( E C M ) operation, a square hole of dimensions 5 mm × mm is drilled in a block of copper. The current used is 5000 A. Atomic weight of copper is 63 and valence of dissolution is 1. Faradays constant is 96500 coulomb. The material removal rate (g/s) is[2008]a)0.326b)3.26c)3.15 × 103d)3.15 × 105Correct answer is option 'B'. Can you explain this answer?.
In a electrochemical machining ( E C M ) operation, a square hole of dimensions 5 mm × mm is drilled in a block of copper. The current used is 5000 A. Atomic weight of copper is 63 and valence of dissolution is 1. Faradays constant is 96500 coulomb. The material removal rate (g/s) is[2008]a)0.326b)3.26c)3.15 × 103d)3.15 × 105Correct answer is option 'B'. Can you explain this answer? for Mechanical Engineering 2024 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about In a electrochemical machining ( E C M ) operation, a square hole of dimensions 5 mm × mm is drilled in a block of copper. The current used is 5000 A. Atomic weight of copper is 63 and valence of dissolution is 1. Faradays constant is 96500 coulomb. The material removal rate (g/s) is[2008]a)0.326b)3.26c)3.15 × 103d)3.15 × 105Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In a electrochemical machining ( E C M ) operation, a square hole of dimensions 5 mm × mm is drilled in a block of copper. The current used is 5000 A. Atomic weight of copper is 63 and valence of dissolution is 1. Faradays constant is 96500 coulomb. The material removal rate (g/s) is[2008]a)0.326b)3.26c)3.15 × 103d)3.15 × 105Correct answer is option 'B'. Can you explain this answer?.
Solutions for In a electrochemical machining ( E C M ) operation, a square hole of dimensions 5 mm × mm is drilled in a block of copper. The current used is 5000 A. Atomic weight of copper is 63 and valence of dissolution is 1. Faradays constant is 96500 coulomb. The material removal rate (g/s) is[2008]a)0.326b)3.26c)3.15 × 103d)3.15 × 105Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Mechanical Engineering.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
Here you can find the meaning of In a electrochemical machining ( E C M ) operation, a square hole of dimensions 5 mm × mm is drilled in a block of copper. The current used is 5000 A. Atomic weight of copper is 63 and valence of dissolution is 1. Faradays constant is 96500 coulomb. The material removal rate (g/s) is[2008]a)0.326b)3.26c)3.15 × 103d)3.15 × 105Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
In a electrochemical machining ( E C M ) operation, a square hole of dimensions 5 mm × mm is drilled in a block of copper. The current used is 5000 A. Atomic weight of copper is 63 and valence of dissolution is 1. Faradays constant is 96500 coulomb. The material removal rate (g/s) is[2008]a)0.326b)3.26c)3.15 × 103d)3.15 × 105Correct answer is option 'B'. Can you explain this answer?, a detailed solution for In a electrochemical machining ( E C M ) operation, a square hole of dimensions 5 mm × mm is drilled in a block of copper. The current used is 5000 A. Atomic weight of copper is 63 and valence of dissolution is 1. Faradays constant is 96500 coulomb. The material removal rate (g/s) is[2008]a)0.326b)3.26c)3.15 × 103d)3.15 × 105Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of In a electrochemical machining ( E C M ) operation, a square hole of dimensions 5 mm × mm is drilled in a block of copper. The current used is 5000 A. Atomic weight of copper is 63 and valence of dissolution is 1. Faradays constant is 96500 coulomb. The material removal rate (g/s) is[2008]a)0.326b)3.26c)3.15 × 103d)3.15 × 105Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice In a electrochemical machining ( E C M ) operation, a square hole of dimensions 5 mm × mm is drilled in a block of copper. The current used is 5000 A. Atomic weight of copper is 63 and valence of dissolution is 1. Faradays constant is 96500 coulomb. The material removal rate (g/s) is[2008]a)0.326b)3.26c)3.15 × 103d)3.15 × 105Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice Mechanical Engineering tests.

|
Explore Courses for Mechanical Engineering exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























