Chemical Engineering Exam > Chemical Engineering Questions > Consider H andO gas, both at temperature of 3...
Start Learning for Free
Consider H and O₂ gas, both at temperature of 30°C. The thermal conductivity of H is ________ than that of O₂.
- a)greater
- b)equal
- c)lower
- d)depends upon pressure
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
Consider H andO gas, both at temperature of 30°C. The thermal cond...
The thermal conductivity of a gaseous medium is dominantly based on diffusion rate, which depends on root mean square velocity.The root mean square velocity for molecules is given by
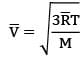
where M is the molecular mass of the gas & T is the temperature of the gas and ̅ is universal gas constant. Thus diffusion rate and therefore thermal conductivity is lower for gases with higher molecular masses because
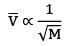
Therefore H₂ gas has more thermal conductivity than O₂.
Most Upvoted Answer
Consider H andO gas, both at temperature of 30°C. The thermal cond...
The thermal conductivity of a gaseous medium is dominantly based on diffusion rate, which depends on root mean square velocity.The root mean square velocity for molecules is given by
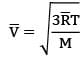
where M is the molecular mass of the gas & T is the temperature of the gas and ̅ is universal gas constant. Thus diffusion rate and therefore thermal conductivity is lower for gases with higher molecular masses because
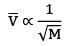
Therefore H₂ gas has more thermal conductivity than O₂.
Free Test
FREE
| Start Free Test |
Community Answer
Consider H andO gas, both at temperature of 30°C. The thermal cond...
Thermal Conductivity of H vs O Gas
Thermal conductivity is a measure of a material's ability to conduct heat. In the case of gases, thermal conductivity is dependent on factors such as molecular weight, speed of molecular movement, and collisions between molecules.
Hydrogen (H) Gas
- Hydrogen gas has a lower molecular weight (2 g/mol) compared to oxygen gas (32 g/mol).
- Due to its lower molecular weight, hydrogen molecules have higher average speeds at a given temperature compared to oxygen molecules.
- The higher speed of hydrogen molecules results in more frequent collisions and interactions, leading to higher thermal conductivity.
Oxygen (O) Gas
- Oxygen gas has a higher molecular weight compared to hydrogen gas.
- Oxygen molecules have lower average speeds at a given temperature compared to hydrogen molecules.
- The lower speed of oxygen molecules results in fewer collisions and interactions, leading to lower thermal conductivity.
Conclusion
Given the differences in molecular weight and molecular speed between hydrogen and oxygen gases, it can be concluded that the thermal conductivity of hydrogen gas is greater than that of oxygen gas at the same temperature.
Thermal conductivity is a measure of a material's ability to conduct heat. In the case of gases, thermal conductivity is dependent on factors such as molecular weight, speed of molecular movement, and collisions between molecules.
Hydrogen (H) Gas
- Hydrogen gas has a lower molecular weight (2 g/mol) compared to oxygen gas (32 g/mol).
- Due to its lower molecular weight, hydrogen molecules have higher average speeds at a given temperature compared to oxygen molecules.
- The higher speed of hydrogen molecules results in more frequent collisions and interactions, leading to higher thermal conductivity.
Oxygen (O) Gas
- Oxygen gas has a higher molecular weight compared to hydrogen gas.
- Oxygen molecules have lower average speeds at a given temperature compared to hydrogen molecules.
- The lower speed of oxygen molecules results in fewer collisions and interactions, leading to lower thermal conductivity.
Conclusion
Given the differences in molecular weight and molecular speed between hydrogen and oxygen gases, it can be concluded that the thermal conductivity of hydrogen gas is greater than that of oxygen gas at the same temperature.

|
Explore Courses for Chemical Engineering exam
|

|
Question Description
Consider H andO gas, both at temperature of 30°C. The thermal conductivity of H is ________ than that ofO.a)greaterb)equalc)lowerd)depends upon pressureCorrect answer is option 'A'. Can you explain this answer? for Chemical Engineering 2025 is part of Chemical Engineering preparation. The Question and answers have been prepared according to the Chemical Engineering exam syllabus. Information about Consider H andO gas, both at temperature of 30°C. The thermal conductivity of H is ________ than that ofO.a)greaterb)equalc)lowerd)depends upon pressureCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Chemical Engineering 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Consider H andO gas, both at temperature of 30°C. The thermal conductivity of H is ________ than that ofO.a)greaterb)equalc)lowerd)depends upon pressureCorrect answer is option 'A'. Can you explain this answer?.
Consider H andO gas, both at temperature of 30°C. The thermal conductivity of H is ________ than that ofO.a)greaterb)equalc)lowerd)depends upon pressureCorrect answer is option 'A'. Can you explain this answer? for Chemical Engineering 2025 is part of Chemical Engineering preparation. The Question and answers have been prepared according to the Chemical Engineering exam syllabus. Information about Consider H andO gas, both at temperature of 30°C. The thermal conductivity of H is ________ than that ofO.a)greaterb)equalc)lowerd)depends upon pressureCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Chemical Engineering 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Consider H andO gas, both at temperature of 30°C. The thermal conductivity of H is ________ than that ofO.a)greaterb)equalc)lowerd)depends upon pressureCorrect answer is option 'A'. Can you explain this answer?.
Solutions for Consider H andO gas, both at temperature of 30°C. The thermal conductivity of H is ________ than that ofO.a)greaterb)equalc)lowerd)depends upon pressureCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemical Engineering.
Download more important topics, notes, lectures and mock test series for Chemical Engineering Exam by signing up for free.
Here you can find the meaning of Consider H andO gas, both at temperature of 30°C. The thermal conductivity of H is ________ than that ofO.a)greaterb)equalc)lowerd)depends upon pressureCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Consider H andO gas, both at temperature of 30°C. The thermal conductivity of H is ________ than that ofO.a)greaterb)equalc)lowerd)depends upon pressureCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for Consider H andO gas, both at temperature of 30°C. The thermal conductivity of H is ________ than that ofO.a)greaterb)equalc)lowerd)depends upon pressureCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of Consider H andO gas, both at temperature of 30°C. The thermal conductivity of H is ________ than that ofO.a)greaterb)equalc)lowerd)depends upon pressureCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Consider H andO gas, both at temperature of 30°C. The thermal conductivity of H is ________ than that ofO.a)greaterb)equalc)lowerd)depends upon pressureCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice Chemical Engineering tests.

|
Explore Courses for Chemical Engineering exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















