Mechanical Engineering Exam > Mechanical Engineering Questions > As the temperature increases, the thermal con...
Start Learning for Free
As the temperature increases, the thermal conductivity of a gas
[2014]
- a)increases
- b)decreases
- c)remains constant
- d)increases up to a certain temperature and then decreases
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
As the temperature increases, the thermal conductivity of a gas[2014]a...
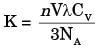
Where K is thermal conductivity
V is mean particle speed
λ is mean free path
CV is molar head capacity
NA is Avogadro's number
n is particles per unit volume
Gases transfer heat by direct collisions between molecules. As the temperature increases, the thermal conductivity increases due to increase in speed, movement and collisions in the molecules.
From the above expression, by increasing mean particle speed, the thermal conductivity increases.
Most Upvoted Answer
As the temperature increases, the thermal conductivity of a gas[2014]a...
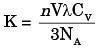
Where K is thermal conductivity
V is mean particle speed
λ is mean free path
CV is molar head capacity
NA is Avogadro's number
n is particles per unit volume
Gases transfer heat by direct collisions between molecules. As the temperature increases, the thermal conductivity increases due to increase in speed, movement and collisions in the molecules.
From the above expression, by increasing mean particle speed, the thermal conductivity increases.
Free Test
FREE
| Start Free Test |
Community Answer
As the temperature increases, the thermal conductivity of a gas[2014]a...
Introduction:
In order to understand why the thermal conductivity of a gas increases as the temperature increases, it is important to first define what thermal conductivity is. Thermal conductivity is a property of a material that describes its ability to conduct heat. It is defined as the amount of heat that can pass through a unit area of a material in a unit time when a temperature gradient exists across the material. In other words, it is a measure of how easily heat can be transferred through a material.
Explanation:
When the temperature of a gas increases, the thermal conductivity also increases. This can be explained by the behavior of gas molecules at different temperatures.
1. Molecular Motion:
At higher temperatures, the molecules in a gas move faster and have higher kinetic energy. This increased molecular motion leads to more frequent collisions between the gas molecules, which in turn increases the number of collisions with neighboring molecules. These collisions transfer energy from one molecule to another, facilitating the conduction of heat.
2. Increased Energy Transfer:
With higher temperatures, the molecules gain more energy, resulting in increased energy transfer between them. As a result, the gas molecules can transfer heat more efficiently, leading to an increase in thermal conductivity.
3. Enhanced Vibrational Modes:
At higher temperatures, the gas molecules can also undergo additional vibrational modes. These additional modes allow the molecules to absorb and transfer more heat, thereby increasing the thermal conductivity of the gas.
4. Decreased Mean Free Path:
Another factor that contributes to the increase in thermal conductivity with temperature is the decrease in the mean free path of the gas molecules. The mean free path is the average distance a molecule travels between collisions. As the temperature increases, the gas molecules move faster and collide more frequently, reducing the mean free path. This reduction in the mean free path allows for more efficient heat transfer through the gas, resulting in higher thermal conductivity.
Conclusion:
In summary, as the temperature of a gas increases, the thermal conductivity also increases. This is due to the increased molecular motion, enhanced energy transfer, additional vibrational modes, and decreased mean free path of the gas molecules at higher temperatures. These factors collectively contribute to a higher thermal conductivity, allowing the gas to conduct heat more effectively.
In order to understand why the thermal conductivity of a gas increases as the temperature increases, it is important to first define what thermal conductivity is. Thermal conductivity is a property of a material that describes its ability to conduct heat. It is defined as the amount of heat that can pass through a unit area of a material in a unit time when a temperature gradient exists across the material. In other words, it is a measure of how easily heat can be transferred through a material.
Explanation:
When the temperature of a gas increases, the thermal conductivity also increases. This can be explained by the behavior of gas molecules at different temperatures.
1. Molecular Motion:
At higher temperatures, the molecules in a gas move faster and have higher kinetic energy. This increased molecular motion leads to more frequent collisions between the gas molecules, which in turn increases the number of collisions with neighboring molecules. These collisions transfer energy from one molecule to another, facilitating the conduction of heat.
2. Increased Energy Transfer:
With higher temperatures, the molecules gain more energy, resulting in increased energy transfer between them. As a result, the gas molecules can transfer heat more efficiently, leading to an increase in thermal conductivity.
3. Enhanced Vibrational Modes:
At higher temperatures, the gas molecules can also undergo additional vibrational modes. These additional modes allow the molecules to absorb and transfer more heat, thereby increasing the thermal conductivity of the gas.
4. Decreased Mean Free Path:
Another factor that contributes to the increase in thermal conductivity with temperature is the decrease in the mean free path of the gas molecules. The mean free path is the average distance a molecule travels between collisions. As the temperature increases, the gas molecules move faster and collide more frequently, reducing the mean free path. This reduction in the mean free path allows for more efficient heat transfer through the gas, resulting in higher thermal conductivity.
Conclusion:
In summary, as the temperature of a gas increases, the thermal conductivity also increases. This is due to the increased molecular motion, enhanced energy transfer, additional vibrational modes, and decreased mean free path of the gas molecules at higher temperatures. These factors collectively contribute to a higher thermal conductivity, allowing the gas to conduct heat more effectively.
Attention Mechanical Engineering Students!
To make sure you are not studying endlessly, EduRev has designed Mechanical Engineering study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Mechanical Engineering.

|
Explore Courses for Mechanical Engineering exam
|

|
Similar Mechanical Engineering Doubts
As the temperature increases, the thermal conductivity of a gas[2014]a)increasesb)decreasesc)remains constantd)increases up to a certain temperature and then decreasesCorrect answer is option 'A'. Can you explain this answer?
Question Description
As the temperature increases, the thermal conductivity of a gas[2014]a)increasesb)decreasesc)remains constantd)increases up to a certain temperature and then decreasesCorrect answer is option 'A'. Can you explain this answer? for Mechanical Engineering 2024 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about As the temperature increases, the thermal conductivity of a gas[2014]a)increasesb)decreasesc)remains constantd)increases up to a certain temperature and then decreasesCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for As the temperature increases, the thermal conductivity of a gas[2014]a)increasesb)decreasesc)remains constantd)increases up to a certain temperature and then decreasesCorrect answer is option 'A'. Can you explain this answer?.
As the temperature increases, the thermal conductivity of a gas[2014]a)increasesb)decreasesc)remains constantd)increases up to a certain temperature and then decreasesCorrect answer is option 'A'. Can you explain this answer? for Mechanical Engineering 2024 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about As the temperature increases, the thermal conductivity of a gas[2014]a)increasesb)decreasesc)remains constantd)increases up to a certain temperature and then decreasesCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for As the temperature increases, the thermal conductivity of a gas[2014]a)increasesb)decreasesc)remains constantd)increases up to a certain temperature and then decreasesCorrect answer is option 'A'. Can you explain this answer?.
Solutions for As the temperature increases, the thermal conductivity of a gas[2014]a)increasesb)decreasesc)remains constantd)increases up to a certain temperature and then decreasesCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Mechanical Engineering.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
Here you can find the meaning of As the temperature increases, the thermal conductivity of a gas[2014]a)increasesb)decreasesc)remains constantd)increases up to a certain temperature and then decreasesCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
As the temperature increases, the thermal conductivity of a gas[2014]a)increasesb)decreasesc)remains constantd)increases up to a certain temperature and then decreasesCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for As the temperature increases, the thermal conductivity of a gas[2014]a)increasesb)decreasesc)remains constantd)increases up to a certain temperature and then decreasesCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of As the temperature increases, the thermal conductivity of a gas[2014]a)increasesb)decreasesc)remains constantd)increases up to a certain temperature and then decreasesCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice As the temperature increases, the thermal conductivity of a gas[2014]a)increasesb)decreasesc)remains constantd)increases up to a certain temperature and then decreasesCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice Mechanical Engineering tests.

|
Explore Courses for Mechanical Engineering exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























