Mechanical Engineering Exam > Mechanical Engineering Questions > Consider the following two processes;I. A hea...
Start Learning for Free
Consider the following two processes;
I. A heat source at 1200 K loses 2500 kJ of heat to a sink at 800 K
II. A heat source at 800 K loses 2000 kJ of heat to a sink at 500 K
Which of the following statements is true?
I. A heat source at 1200 K loses 2500 kJ of heat to a sink at 800 K
II. A heat source at 800 K loses 2000 kJ of heat to a sink at 500 K
Which of the following statements is true?
[2010]
- a)Process I is more irreversible than Process II
- b)Process II is more irreversible than Process I
- c)Irreversibility associated in both the processes are equal
- d)Both the processes are reversible
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Consider the following two processes;I. A heat source at 1200 K loses ...
From Clausius inequality,
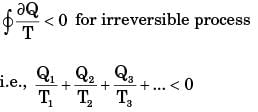
For process I
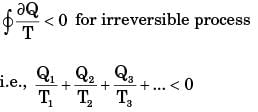
For process I
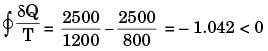
For process II
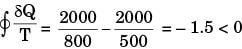
Hence process II is more irreversible than process I.
Most Upvoted Answer
Consider the following two processes;I. A heat source at 1200 K loses ...
Process I:
- Heat source temperature: 1200 K
- Heat loss: 2500 kJ
- Sink temperature: 800 K
Process II:
- Heat source temperature: 800 K
- Heat loss: 2000 kJ
- Sink temperature: 500 K
Understanding Irreversibility:
Irreversibility is a measure of the inefficiency of a process. It indicates the extent to which a process deviates from being reversible, meaning that it cannot be undone without leaving some trace on the surroundings. Irreversibilities are associated with factors such as heat transfer across a finite temperature difference, friction, unrestrained expansion, etc.
Comparing the Irreversibility of Processes I and II:
To compare the irreversibility of the two processes, we can consider the efficiency of each process. The efficiency is defined as the ratio of the work output to the heat input. In this case, since there is no work output mentioned, we can use the heat loss as a measure of inefficiency.
Process I:
- Heat loss = 2500 kJ
- Temperature difference = 1200 K - 800 K = 400 K
Process II:
- Heat loss = 2000 kJ
- Temperature difference = 800 K - 500 K = 300 K
Comparing the Temperature Differences:
The temperature difference between the heat source and sink is an important factor in determining the irreversibility of a process. A larger temperature difference generally indicates a higher degree of irreversibility.
In this case, the temperature difference for Process I is 400 K, while for Process II it is 300 K. Since the temperature difference for Process I is greater, we can conclude that Process I is more irreversible than Process II.
Conclusion:
Based on the comparison of temperature differences, we can conclude that Process I is more irreversible than Process II. Therefore, the correct answer is option 'B'.
- Heat source temperature: 1200 K
- Heat loss: 2500 kJ
- Sink temperature: 800 K
Process II:
- Heat source temperature: 800 K
- Heat loss: 2000 kJ
- Sink temperature: 500 K
Understanding Irreversibility:
Irreversibility is a measure of the inefficiency of a process. It indicates the extent to which a process deviates from being reversible, meaning that it cannot be undone without leaving some trace on the surroundings. Irreversibilities are associated with factors such as heat transfer across a finite temperature difference, friction, unrestrained expansion, etc.
Comparing the Irreversibility of Processes I and II:
To compare the irreversibility of the two processes, we can consider the efficiency of each process. The efficiency is defined as the ratio of the work output to the heat input. In this case, since there is no work output mentioned, we can use the heat loss as a measure of inefficiency.
Process I:
- Heat loss = 2500 kJ
- Temperature difference = 1200 K - 800 K = 400 K
Process II:
- Heat loss = 2000 kJ
- Temperature difference = 800 K - 500 K = 300 K
Comparing the Temperature Differences:
The temperature difference between the heat source and sink is an important factor in determining the irreversibility of a process. A larger temperature difference generally indicates a higher degree of irreversibility.
In this case, the temperature difference for Process I is 400 K, while for Process II it is 300 K. Since the temperature difference for Process I is greater, we can conclude that Process I is more irreversible than Process II.
Conclusion:
Based on the comparison of temperature differences, we can conclude that Process I is more irreversible than Process II. Therefore, the correct answer is option 'B'.
Free Test
| FREE | Start Free Test |
Community Answer
Consider the following two processes;I. A heat source at 1200 K loses ...
From Clausius inequality,
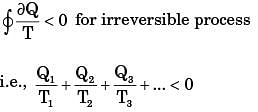
For process I
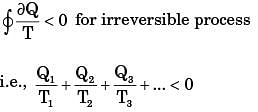
For process I
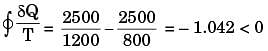
For process II
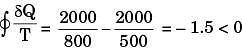
Hence process II is more irreversible than process I.
Attention Mechanical Engineering Students!
To make sure you are not studying endlessly, EduRev has designed Mechanical Engineering study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Mechanical Engineering.

|
Explore Courses for Mechanical Engineering exam
|

|
Similar Mechanical Engineering Doubts
Consider the following two processes;I. A heat source at 1200 K loses 2500 kJ of heat to a sink at 800 KII. A heat source at 800 K loses 2000 kJ of heat to a sink at 500 KWhich of the following statements is true?[2010]a)Process I is more irreversible than Process IIb)Process II is more irreversible than Process Ic)Irreversibility associated in both the processes are equald)Both the processes are reversibleCorrect answer is option 'B'. Can you explain this answer?
Question Description
Consider the following two processes;I. A heat source at 1200 K loses 2500 kJ of heat to a sink at 800 KII. A heat source at 800 K loses 2000 kJ of heat to a sink at 500 KWhich of the following statements is true?[2010]a)Process I is more irreversible than Process IIb)Process II is more irreversible than Process Ic)Irreversibility associated in both the processes are equald)Both the processes are reversibleCorrect answer is option 'B'. Can you explain this answer? for Mechanical Engineering 2024 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about Consider the following two processes;I. A heat source at 1200 K loses 2500 kJ of heat to a sink at 800 KII. A heat source at 800 K loses 2000 kJ of heat to a sink at 500 KWhich of the following statements is true?[2010]a)Process I is more irreversible than Process IIb)Process II is more irreversible than Process Ic)Irreversibility associated in both the processes are equald)Both the processes are reversibleCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Consider the following two processes;I. A heat source at 1200 K loses 2500 kJ of heat to a sink at 800 KII. A heat source at 800 K loses 2000 kJ of heat to a sink at 500 KWhich of the following statements is true?[2010]a)Process I is more irreversible than Process IIb)Process II is more irreversible than Process Ic)Irreversibility associated in both the processes are equald)Both the processes are reversibleCorrect answer is option 'B'. Can you explain this answer?.
Consider the following two processes;I. A heat source at 1200 K loses 2500 kJ of heat to a sink at 800 KII. A heat source at 800 K loses 2000 kJ of heat to a sink at 500 KWhich of the following statements is true?[2010]a)Process I is more irreversible than Process IIb)Process II is more irreversible than Process Ic)Irreversibility associated in both the processes are equald)Both the processes are reversibleCorrect answer is option 'B'. Can you explain this answer? for Mechanical Engineering 2024 is part of Mechanical Engineering preparation. The Question and answers have been prepared according to the Mechanical Engineering exam syllabus. Information about Consider the following two processes;I. A heat source at 1200 K loses 2500 kJ of heat to a sink at 800 KII. A heat source at 800 K loses 2000 kJ of heat to a sink at 500 KWhich of the following statements is true?[2010]a)Process I is more irreversible than Process IIb)Process II is more irreversible than Process Ic)Irreversibility associated in both the processes are equald)Both the processes are reversibleCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Mechanical Engineering 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Consider the following two processes;I. A heat source at 1200 K loses 2500 kJ of heat to a sink at 800 KII. A heat source at 800 K loses 2000 kJ of heat to a sink at 500 KWhich of the following statements is true?[2010]a)Process I is more irreversible than Process IIb)Process II is more irreversible than Process Ic)Irreversibility associated in both the processes are equald)Both the processes are reversibleCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Consider the following two processes;I. A heat source at 1200 K loses 2500 kJ of heat to a sink at 800 KII. A heat source at 800 K loses 2000 kJ of heat to a sink at 500 KWhich of the following statements is true?[2010]a)Process I is more irreversible than Process IIb)Process II is more irreversible than Process Ic)Irreversibility associated in both the processes are equald)Both the processes are reversibleCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Mechanical Engineering.
Download more important topics, notes, lectures and mock test series for Mechanical Engineering Exam by signing up for free.
Here you can find the meaning of Consider the following two processes;I. A heat source at 1200 K loses 2500 kJ of heat to a sink at 800 KII. A heat source at 800 K loses 2000 kJ of heat to a sink at 500 KWhich of the following statements is true?[2010]a)Process I is more irreversible than Process IIb)Process II is more irreversible than Process Ic)Irreversibility associated in both the processes are equald)Both the processes are reversibleCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Consider the following two processes;I. A heat source at 1200 K loses 2500 kJ of heat to a sink at 800 KII. A heat source at 800 K loses 2000 kJ of heat to a sink at 500 KWhich of the following statements is true?[2010]a)Process I is more irreversible than Process IIb)Process II is more irreversible than Process Ic)Irreversibility associated in both the processes are equald)Both the processes are reversibleCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Consider the following two processes;I. A heat source at 1200 K loses 2500 kJ of heat to a sink at 800 KII. A heat source at 800 K loses 2000 kJ of heat to a sink at 500 KWhich of the following statements is true?[2010]a)Process I is more irreversible than Process IIb)Process II is more irreversible than Process Ic)Irreversibility associated in both the processes are equald)Both the processes are reversibleCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Consider the following two processes;I. A heat source at 1200 K loses 2500 kJ of heat to a sink at 800 KII. A heat source at 800 K loses 2000 kJ of heat to a sink at 500 KWhich of the following statements is true?[2010]a)Process I is more irreversible than Process IIb)Process II is more irreversible than Process Ic)Irreversibility associated in both the processes are equald)Both the processes are reversibleCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Consider the following two processes;I. A heat source at 1200 K loses 2500 kJ of heat to a sink at 800 KII. A heat source at 800 K loses 2000 kJ of heat to a sink at 500 KWhich of the following statements is true?[2010]a)Process I is more irreversible than Process IIb)Process II is more irreversible than Process Ic)Irreversibility associated in both the processes are equald)Both the processes are reversibleCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice Mechanical Engineering tests.

|
Explore Courses for Mechanical Engineering exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























