JEE Exam > JEE Questions > Which of the following represents Schotten Ba...
Start Learning for Free
Which of the following represents Schotten Baumann reaction?
- a)formation of amides from amines and acid chlorides/NaOH
- b)for mation of amines from amides an d LiAlH4
- c)formation of amines from amides and Br2/NaOH
- d)formation of amides from oxime and H2SO4
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
Which of the following represents Schotten Baumann reaction?a)formatio...
Schotten-Baumann Conditions
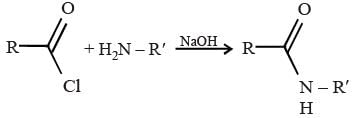
The use of added base to drive the equilibrium in the formation of amides from amines and acid chlorides.

The use of added base to drive the equilibrium in the formation of amides from amines and acid chlorides.
Most Upvoted Answer
Which of the following represents Schotten Baumann reaction?a)formatio...
Schotten Baumann Reaction
The Schotten Baumann reaction is a chemical reaction that is used to form amides from amines and acid chlorides. The reaction involves the use of sodium hydroxide as a base, which serves to neutralize the hydrogen chloride generated during the reaction.
Mechanism
The Schotten Baumann reaction proceeds through the following mechanism:
Step 1: Deprotonation
The amine is deprotonated by the base to generate an amide ion.
Step 2: Nucleophilic attack
The amide ion attacks the acid chloride to form an intermediate.
Step 3: Protonation
The intermediate is then protonated by the acid chloride to form the final amide product.
Overall Reaction
The overall reaction for the Schotten Baumann reaction is:
amine + acid chloride + NaOH → amide + NaCl + H2O
Applications
The Schotten Baumann reaction is widely used in organic chemistry for the synthesis of amides, which are important intermediates in the production of pharmaceuticals, agrochemicals, and other fine chemicals.
Conclusion
In conclusion, the Schotten Baumann reaction is a valuable tool for the synthesis of amides from amines and acid chlorides. The reaction proceeds through a straightforward mechanism and has broad synthetic applications.
The Schotten Baumann reaction is a chemical reaction that is used to form amides from amines and acid chlorides. The reaction involves the use of sodium hydroxide as a base, which serves to neutralize the hydrogen chloride generated during the reaction.
Mechanism
The Schotten Baumann reaction proceeds through the following mechanism:
Step 1: Deprotonation
The amine is deprotonated by the base to generate an amide ion.
Step 2: Nucleophilic attack
The amide ion attacks the acid chloride to form an intermediate.
Step 3: Protonation
The intermediate is then protonated by the acid chloride to form the final amide product.
Overall Reaction
The overall reaction for the Schotten Baumann reaction is:
amine + acid chloride + NaOH → amide + NaCl + H2O
Applications
The Schotten Baumann reaction is widely used in organic chemistry for the synthesis of amides, which are important intermediates in the production of pharmaceuticals, agrochemicals, and other fine chemicals.
Conclusion
In conclusion, the Schotten Baumann reaction is a valuable tool for the synthesis of amides from amines and acid chlorides. The reaction proceeds through a straightforward mechanism and has broad synthetic applications.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Which of the following represents Schotten Baumann reaction?a)formation of amides from amines and acid chlorides/NaOHb)for mation of amines from amides an d LiAlH4c)formation of amines from amides and Br2/NaOHd)formation of amides from oxime and H2SO4Correct answer is option 'A'. Can you explain this answer?
Question Description
Which of the following represents Schotten Baumann reaction?a)formation of amides from amines and acid chlorides/NaOHb)for mation of amines from amides an d LiAlH4c)formation of amines from amides and Br2/NaOHd)formation of amides from oxime and H2SO4Correct answer is option 'A'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Which of the following represents Schotten Baumann reaction?a)formation of amides from amines and acid chlorides/NaOHb)for mation of amines from amides an d LiAlH4c)formation of amines from amides and Br2/NaOHd)formation of amides from oxime and H2SO4Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following represents Schotten Baumann reaction?a)formation of amides from amines and acid chlorides/NaOHb)for mation of amines from amides an d LiAlH4c)formation of amines from amides and Br2/NaOHd)formation of amides from oxime and H2SO4Correct answer is option 'A'. Can you explain this answer?.
Which of the following represents Schotten Baumann reaction?a)formation of amides from amines and acid chlorides/NaOHb)for mation of amines from amides an d LiAlH4c)formation of amines from amides and Br2/NaOHd)formation of amides from oxime and H2SO4Correct answer is option 'A'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Which of the following represents Schotten Baumann reaction?a)formation of amides from amines and acid chlorides/NaOHb)for mation of amines from amides an d LiAlH4c)formation of amines from amides and Br2/NaOHd)formation of amides from oxime and H2SO4Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following represents Schotten Baumann reaction?a)formation of amides from amines and acid chlorides/NaOHb)for mation of amines from amides an d LiAlH4c)formation of amines from amides and Br2/NaOHd)formation of amides from oxime and H2SO4Correct answer is option 'A'. Can you explain this answer?.
Solutions for Which of the following represents Schotten Baumann reaction?a)formation of amides from amines and acid chlorides/NaOHb)for mation of amines from amides an d LiAlH4c)formation of amines from amides and Br2/NaOHd)formation of amides from oxime and H2SO4Correct answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Which of the following represents Schotten Baumann reaction?a)formation of amides from amines and acid chlorides/NaOHb)for mation of amines from amides an d LiAlH4c)formation of amines from amides and Br2/NaOHd)formation of amides from oxime and H2SO4Correct answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following represents Schotten Baumann reaction?a)formation of amides from amines and acid chlorides/NaOHb)for mation of amines from amides an d LiAlH4c)formation of amines from amides and Br2/NaOHd)formation of amides from oxime and H2SO4Correct answer is option 'A'. Can you explain this answer?, a detailed solution for Which of the following represents Schotten Baumann reaction?a)formation of amides from amines and acid chlorides/NaOHb)for mation of amines from amides an d LiAlH4c)formation of amines from amides and Br2/NaOHd)formation of amides from oxime and H2SO4Correct answer is option 'A'. Can you explain this answer? has been provided alongside types of Which of the following represents Schotten Baumann reaction?a)formation of amides from amines and acid chlorides/NaOHb)for mation of amines from amides an d LiAlH4c)formation of amines from amides and Br2/NaOHd)formation of amides from oxime and H2SO4Correct answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following represents Schotten Baumann reaction?a)formation of amides from amines and acid chlorides/NaOHb)for mation of amines from amides an d LiAlH4c)formation of amines from amides and Br2/NaOHd)formation of amides from oxime and H2SO4Correct answer is option 'A'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























