Class 12 Exam > Class 12 Questions > Directions : Read the passage given below and...
Start Learning for Free
Directions : Read the passage given below and answer the following questions:
Raoult’s law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton’s law of partial pressure states that the total pressure (ptotal) over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as :
Ptotal = P1 + P2
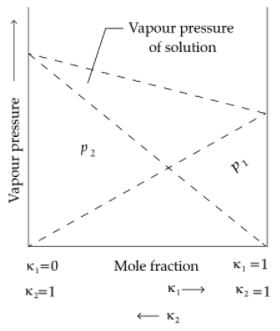
Raoult’s law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton’s law of partial pressure states that the total pressure (ptotal) over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as :
Ptotal = P1 + P2

Q. In comparison to a 0.01 M solution of glucose, the depression in freezing point of a 0.01 M MgCl2 solution is ________.
- a)the same
- b)about twice
- c)about three times
- d)about six times
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
Directions : Read the passage given below and answer the following que...
ΔTf =iKf m, where i = 1 for glucose.
ΔTf glucose = 1 × Kf × 0.01
In case of MgCl2 → Mg2+ + 2Cl−, where i = 3,
∅TfMgCl2 = 3 × 0.01 × Kf
⇒ ∅TfMgCl2 = 3 × ΔTf glucose
Hence, the depression in freezing point of MgCl2 is three times that of glucose.
ΔTf glucose = 1 × Kf × 0.01
In case of MgCl2 → Mg2+ + 2Cl−, where i = 3,
∅TfMgCl2 = 3 × 0.01 × Kf
⇒ ∅TfMgCl2 = 3 × ΔTf glucose
Hence, the depression in freezing point of MgCl2 is three times that of glucose.
Free Test
FREE
| Start Free Test |
Community Answer
Directions : Read the passage given below and answer the following que...
ΔTf =iKf m, where i = 1 for glucose.
ΔTf glucose = 1 × Kf × 0.01
In case of MgCl2 → Mg2+ + 2Cl−, where i = 3,
∅TfMgCl2 = 3 × 0.01 × Kf
⇒ ∅TfMgCl2 = 3 × ΔTf glucose
Hence, the depression in freezing point of MgCl2 is three times that of glucose.
ΔTf glucose = 1 × Kf × 0.01
In case of MgCl2 → Mg2+ + 2Cl−, where i = 3,
∅TfMgCl2 = 3 × 0.01 × Kf
⇒ ∅TfMgCl2 = 3 × ΔTf glucose
Hence, the depression in freezing point of MgCl2 is three times that of glucose.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Directions : Read the passage given below and answer the following questions:Raoult’s law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton’s law of partial pressure states that the total pressure (ptotal) over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as :Ptotal = P1 + P2Q.In comparison to a 0.01 M solution of glucose, the depression in freezing point of a 0.01 M MgCl2 solution is ________.a)the sameb)about twicec)about three timesd)about six timesCorrect answer is option 'C'. Can you explain this answer?
Question Description
Directions : Read the passage given below and answer the following questions:Raoult’s law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton’s law of partial pressure states that the total pressure (ptotal) over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as :Ptotal = P1 + P2Q.In comparison to a 0.01 M solution of glucose, the depression in freezing point of a 0.01 M MgCl2 solution is ________.a)the sameb)about twicec)about three timesd)about six timesCorrect answer is option 'C'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Directions : Read the passage given below and answer the following questions:Raoult’s law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton’s law of partial pressure states that the total pressure (ptotal) over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as :Ptotal = P1 + P2Q.In comparison to a 0.01 M solution of glucose, the depression in freezing point of a 0.01 M MgCl2 solution is ________.a)the sameb)about twicec)about three timesd)about six timesCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions : Read the passage given below and answer the following questions:Raoult’s law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton’s law of partial pressure states that the total pressure (ptotal) over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as :Ptotal = P1 + P2Q.In comparison to a 0.01 M solution of glucose, the depression in freezing point of a 0.01 M MgCl2 solution is ________.a)the sameb)about twicec)about three timesd)about six timesCorrect answer is option 'C'. Can you explain this answer?.
Directions : Read the passage given below and answer the following questions:Raoult’s law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton’s law of partial pressure states that the total pressure (ptotal) over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as :Ptotal = P1 + P2Q.In comparison to a 0.01 M solution of glucose, the depression in freezing point of a 0.01 M MgCl2 solution is ________.a)the sameb)about twicec)about three timesd)about six timesCorrect answer is option 'C'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Directions : Read the passage given below and answer the following questions:Raoult’s law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton’s law of partial pressure states that the total pressure (ptotal) over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as :Ptotal = P1 + P2Q.In comparison to a 0.01 M solution of glucose, the depression in freezing point of a 0.01 M MgCl2 solution is ________.a)the sameb)about twicec)about three timesd)about six timesCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions : Read the passage given below and answer the following questions:Raoult’s law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton’s law of partial pressure states that the total pressure (ptotal) over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as :Ptotal = P1 + P2Q.In comparison to a 0.01 M solution of glucose, the depression in freezing point of a 0.01 M MgCl2 solution is ________.a)the sameb)about twicec)about three timesd)about six timesCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Directions : Read the passage given below and answer the following questions:Raoult’s law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton’s law of partial pressure states that the total pressure (ptotal) over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as :Ptotal = P1 + P2Q.In comparison to a 0.01 M solution of glucose, the depression in freezing point of a 0.01 M MgCl2 solution is ________.a)the sameb)about twicec)about three timesd)about six timesCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Directions : Read the passage given below and answer the following questions:Raoult’s law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton’s law of partial pressure states that the total pressure (ptotal) over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as :Ptotal = P1 + P2Q.In comparison to a 0.01 M solution of glucose, the depression in freezing point of a 0.01 M MgCl2 solution is ________.a)the sameb)about twicec)about three timesd)about six timesCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Directions : Read the passage given below and answer the following questions:Raoult’s law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton’s law of partial pressure states that the total pressure (ptotal) over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as :Ptotal = P1 + P2Q.In comparison to a 0.01 M solution of glucose, the depression in freezing point of a 0.01 M MgCl2 solution is ________.a)the sameb)about twicec)about three timesd)about six timesCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Directions : Read the passage given below and answer the following questions:Raoult’s law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton’s law of partial pressure states that the total pressure (ptotal) over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as :Ptotal = P1 + P2Q.In comparison to a 0.01 M solution of glucose, the depression in freezing point of a 0.01 M MgCl2 solution is ________.a)the sameb)about twicec)about three timesd)about six timesCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Directions : Read the passage given below and answer the following questions:Raoult’s law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton’s law of partial pressure states that the total pressure (ptotal) over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as :Ptotal = P1 + P2Q.In comparison to a 0.01 M solution of glucose, the depression in freezing point of a 0.01 M MgCl2 solution is ________.a)the sameb)about twicec)about three timesd)about six timesCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Directions : Read the passage given below and answer the following questions:Raoult’s law states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Dalton’s law of partial pressure states that the total pressure (ptotal) over the solution phase in the container will be the sum of the partial pressures of the components of the solution and is given as :Ptotal = P1 + P2Q.In comparison to a 0.01 M solution of glucose, the depression in freezing point of a 0.01 M MgCl2 solution is ________.a)the sameb)about twicec)about three timesd)about six timesCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















