Class 12 Exam > Class 12 Questions > Read the passage given below and answer the ...
Start Learning for Free
Read the passage given below and answer the following questions:
All chemical reactions involve interaction of atoms and molecules. A large number of atoms/molecules are present in a few gram of any chemical compound varying with their atomic/molecular masses. To handle such large number conveniently, the mole concept was introduced. All electrochemical cell reactions are also based on mole concept. For example, a 4.0 molar aqueous solution of NaCl is prepared and 500 mL of this solution is electrolysed. This leads to the evolution of chlorine gas at one of the electrode. The amount of products formed can be calculated by using mole concept.
The following questions are multiple choice questions. Choose the most appropriate answer:
Q. The total number of moles of chlorine gas evolved is
- a)0.5
- b)1.0
- c)1.5
- d)1.9
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Read the passage given below and answer the following questions:All c...
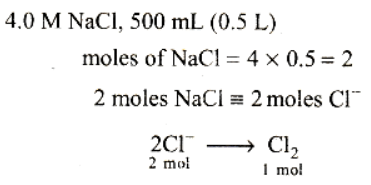
Most Upvoted Answer
Read the passage given below and answer the following questions:All c...
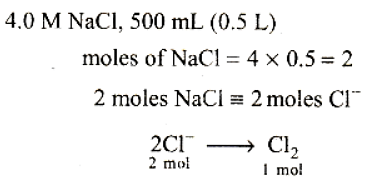
Free Test
FREE
| Start Free Test |
Community Answer
Read the passage given below and answer the following questions:All c...
The given passage discusses the mole concept and its application in electrochemical cell reactions. In one example, a 4.0 molar aqueous solution of NaCl is electrolyzed, resulting in the evolution of chlorine gas. We need to determine the total number of moles of chlorine gas evolved.
To calculate the number of moles of chlorine gas, we need to use the given information about the molarity of the solution and the volume of the solution electrolyzed.
First, let's understand the concept of molarity. Molarity is a measure of the concentration of a solute in a solution and is defined as the number of moles of solute per liter of solution. In this case, the molarity of the NaCl solution is given as 4.0 mol/L.
We are given that 500 mL (or 0.5 L) of the solution is electrolyzed. To calculate the number of moles of chlorine gas evolved, we can use the equation:
moles = molarity × volume (in liters)
Plugging in the values, we have:
moles = 4.0 mol/L × 0.5 L
moles = 2.0 mol
Therefore, the total number of moles of chlorine gas evolved is 2.0 mol.
The correct answer is option B) 1.0 mol.
To summarize:
- Molarity is the measure of concentration of a solute in a solution, defined as the number of moles of solute per liter of solution.
- The molarity of the NaCl solution is given as 4.0 mol/L.
- The volume of the solution electrolyzed is 500 mL or 0.5 L.
- To calculate the number of moles of chlorine gas evolved, we use the equation: moles = molarity × volume.
- Plugging in the values, we find that the total number of moles of chlorine gas evolved is 2.0 mol.
- The correct answer is option B) 1.0 mol.
This explanation provides a detailed understanding of the mole concept, molarity, and how to calculate the number of moles of a substance using the given information.
To calculate the number of moles of chlorine gas, we need to use the given information about the molarity of the solution and the volume of the solution electrolyzed.
First, let's understand the concept of molarity. Molarity is a measure of the concentration of a solute in a solution and is defined as the number of moles of solute per liter of solution. In this case, the molarity of the NaCl solution is given as 4.0 mol/L.
We are given that 500 mL (or 0.5 L) of the solution is electrolyzed. To calculate the number of moles of chlorine gas evolved, we can use the equation:
moles = molarity × volume (in liters)
Plugging in the values, we have:
moles = 4.0 mol/L × 0.5 L
moles = 2.0 mol
Therefore, the total number of moles of chlorine gas evolved is 2.0 mol.
The correct answer is option B) 1.0 mol.
To summarize:
- Molarity is the measure of concentration of a solute in a solution, defined as the number of moles of solute per liter of solution.
- The molarity of the NaCl solution is given as 4.0 mol/L.
- The volume of the solution electrolyzed is 500 mL or 0.5 L.
- To calculate the number of moles of chlorine gas evolved, we use the equation: moles = molarity × volume.
- Plugging in the values, we find that the total number of moles of chlorine gas evolved is 2.0 mol.
- The correct answer is option B) 1.0 mol.
This explanation provides a detailed understanding of the mole concept, molarity, and how to calculate the number of moles of a substance using the given information.

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Read the passage given below and answer the following questions:All chemical reactions involve interaction of atoms and molecules. A large number of atoms/molecules are present in a few gram of any chemical compound varying with their atomic/molecular masses. To handle such large number conveniently, the mole concept was introduced. All electrochemical cell reactions are also based on mole concept. For example, a 4.0 molar aqueous solution of NaCl is prepared and 500 mL of this solution is electrolysed. This leads to the evolution of chlorine gas at one of the electrode. The amount of products formed can be calculated by using mole concept.The following questions are multiple choice questions. Choose the most appropriate answer:Q. The total number of moles of chlorine gas evolved isa)0.5b)1.0c)1.5d)1.9Correct answer is option 'B'. Can you explain this answer?
Question Description
Read the passage given below and answer the following questions:All chemical reactions involve interaction of atoms and molecules. A large number of atoms/molecules are present in a few gram of any chemical compound varying with their atomic/molecular masses. To handle such large number conveniently, the mole concept was introduced. All electrochemical cell reactions are also based on mole concept. For example, a 4.0 molar aqueous solution of NaCl is prepared and 500 mL of this solution is electrolysed. This leads to the evolution of chlorine gas at one of the electrode. The amount of products formed can be calculated by using mole concept.The following questions are multiple choice questions. Choose the most appropriate answer:Q. The total number of moles of chlorine gas evolved isa)0.5b)1.0c)1.5d)1.9Correct answer is option 'B'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Read the passage given below and answer the following questions:All chemical reactions involve interaction of atoms and molecules. A large number of atoms/molecules are present in a few gram of any chemical compound varying with their atomic/molecular masses. To handle such large number conveniently, the mole concept was introduced. All electrochemical cell reactions are also based on mole concept. For example, a 4.0 molar aqueous solution of NaCl is prepared and 500 mL of this solution is electrolysed. This leads to the evolution of chlorine gas at one of the electrode. The amount of products formed can be calculated by using mole concept.The following questions are multiple choice questions. Choose the most appropriate answer:Q. The total number of moles of chlorine gas evolved isa)0.5b)1.0c)1.5d)1.9Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Read the passage given below and answer the following questions:All chemical reactions involve interaction of atoms and molecules. A large number of atoms/molecules are present in a few gram of any chemical compound varying with their atomic/molecular masses. To handle such large number conveniently, the mole concept was introduced. All electrochemical cell reactions are also based on mole concept. For example, a 4.0 molar aqueous solution of NaCl is prepared and 500 mL of this solution is electrolysed. This leads to the evolution of chlorine gas at one of the electrode. The amount of products formed can be calculated by using mole concept.The following questions are multiple choice questions. Choose the most appropriate answer:Q. The total number of moles of chlorine gas evolved isa)0.5b)1.0c)1.5d)1.9Correct answer is option 'B'. Can you explain this answer?.
Read the passage given below and answer the following questions:All chemical reactions involve interaction of atoms and molecules. A large number of atoms/molecules are present in a few gram of any chemical compound varying with their atomic/molecular masses. To handle such large number conveniently, the mole concept was introduced. All electrochemical cell reactions are also based on mole concept. For example, a 4.0 molar aqueous solution of NaCl is prepared and 500 mL of this solution is electrolysed. This leads to the evolution of chlorine gas at one of the electrode. The amount of products formed can be calculated by using mole concept.The following questions are multiple choice questions. Choose the most appropriate answer:Q. The total number of moles of chlorine gas evolved isa)0.5b)1.0c)1.5d)1.9Correct answer is option 'B'. Can you explain this answer? for Class 12 2024 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Read the passage given below and answer the following questions:All chemical reactions involve interaction of atoms and molecules. A large number of atoms/molecules are present in a few gram of any chemical compound varying with their atomic/molecular masses. To handle such large number conveniently, the mole concept was introduced. All electrochemical cell reactions are also based on mole concept. For example, a 4.0 molar aqueous solution of NaCl is prepared and 500 mL of this solution is electrolysed. This leads to the evolution of chlorine gas at one of the electrode. The amount of products formed can be calculated by using mole concept.The following questions are multiple choice questions. Choose the most appropriate answer:Q. The total number of moles of chlorine gas evolved isa)0.5b)1.0c)1.5d)1.9Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 12 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Read the passage given below and answer the following questions:All chemical reactions involve interaction of atoms and molecules. A large number of atoms/molecules are present in a few gram of any chemical compound varying with their atomic/molecular masses. To handle such large number conveniently, the mole concept was introduced. All electrochemical cell reactions are also based on mole concept. For example, a 4.0 molar aqueous solution of NaCl is prepared and 500 mL of this solution is electrolysed. This leads to the evolution of chlorine gas at one of the electrode. The amount of products formed can be calculated by using mole concept.The following questions are multiple choice questions. Choose the most appropriate answer:Q. The total number of moles of chlorine gas evolved isa)0.5b)1.0c)1.5d)1.9Correct answer is option 'B'. Can you explain this answer?.
Solutions for Read the passage given below and answer the following questions:All chemical reactions involve interaction of atoms and molecules. A large number of atoms/molecules are present in a few gram of any chemical compound varying with their atomic/molecular masses. To handle such large number conveniently, the mole concept was introduced. All electrochemical cell reactions are also based on mole concept. For example, a 4.0 molar aqueous solution of NaCl is prepared and 500 mL of this solution is electrolysed. This leads to the evolution of chlorine gas at one of the electrode. The amount of products formed can be calculated by using mole concept.The following questions are multiple choice questions. Choose the most appropriate answer:Q. The total number of moles of chlorine gas evolved isa)0.5b)1.0c)1.5d)1.9Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Read the passage given below and answer the following questions:All chemical reactions involve interaction of atoms and molecules. A large number of atoms/molecules are present in a few gram of any chemical compound varying with their atomic/molecular masses. To handle such large number conveniently, the mole concept was introduced. All electrochemical cell reactions are also based on mole concept. For example, a 4.0 molar aqueous solution of NaCl is prepared and 500 mL of this solution is electrolysed. This leads to the evolution of chlorine gas at one of the electrode. The amount of products formed can be calculated by using mole concept.The following questions are multiple choice questions. Choose the most appropriate answer:Q. The total number of moles of chlorine gas evolved isa)0.5b)1.0c)1.5d)1.9Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Read the passage given below and answer the following questions:All chemical reactions involve interaction of atoms and molecules. A large number of atoms/molecules are present in a few gram of any chemical compound varying with their atomic/molecular masses. To handle such large number conveniently, the mole concept was introduced. All electrochemical cell reactions are also based on mole concept. For example, a 4.0 molar aqueous solution of NaCl is prepared and 500 mL of this solution is electrolysed. This leads to the evolution of chlorine gas at one of the electrode. The amount of products formed can be calculated by using mole concept.The following questions are multiple choice questions. Choose the most appropriate answer:Q. The total number of moles of chlorine gas evolved isa)0.5b)1.0c)1.5d)1.9Correct answer is option 'B'. Can you explain this answer?, a detailed solution for Read the passage given below and answer the following questions:All chemical reactions involve interaction of atoms and molecules. A large number of atoms/molecules are present in a few gram of any chemical compound varying with their atomic/molecular masses. To handle such large number conveniently, the mole concept was introduced. All electrochemical cell reactions are also based on mole concept. For example, a 4.0 molar aqueous solution of NaCl is prepared and 500 mL of this solution is electrolysed. This leads to the evolution of chlorine gas at one of the electrode. The amount of products formed can be calculated by using mole concept.The following questions are multiple choice questions. Choose the most appropriate answer:Q. The total number of moles of chlorine gas evolved isa)0.5b)1.0c)1.5d)1.9Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of Read the passage given below and answer the following questions:All chemical reactions involve interaction of atoms and molecules. A large number of atoms/molecules are present in a few gram of any chemical compound varying with their atomic/molecular masses. To handle such large number conveniently, the mole concept was introduced. All electrochemical cell reactions are also based on mole concept. For example, a 4.0 molar aqueous solution of NaCl is prepared and 500 mL of this solution is electrolysed. This leads to the evolution of chlorine gas at one of the electrode. The amount of products formed can be calculated by using mole concept.The following questions are multiple choice questions. Choose the most appropriate answer:Q. The total number of moles of chlorine gas evolved isa)0.5b)1.0c)1.5d)1.9Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Read the passage given below and answer the following questions:All chemical reactions involve interaction of atoms and molecules. A large number of atoms/molecules are present in a few gram of any chemical compound varying with their atomic/molecular masses. To handle such large number conveniently, the mole concept was introduced. All electrochemical cell reactions are also based on mole concept. For example, a 4.0 molar aqueous solution of NaCl is prepared and 500 mL of this solution is electrolysed. This leads to the evolution of chlorine gas at one of the electrode. The amount of products formed can be calculated by using mole concept.The following questions are multiple choice questions. Choose the most appropriate answer:Q. The total number of moles of chlorine gas evolved isa)0.5b)1.0c)1.5d)1.9Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















