Railways Exam > Railways Questions > Which of the following is incorrect regarding...
Start Learning for Free
Which of the following is incorrect regarding the first law of thermodynamics?
- a)It is a restatement of the principle of conservation of energy
- b)It is applicable to the cyclic process
- c)It introduces the concept of entropy
- d)It introduces the concept of internal energy
Correct answer is option 'C'. Can you explain this answer?
Most Upvoted Answer
Which of the following is incorrect regarding the first law of thermod...
The first law of thermodynamics is a restatement of the law of conservation of energy. It states that energy cannot be created or destroyed in an isolated system; energy can only be transferred or changed from one form to another.
When heat energy is supplied to a gas, two things may occur:
- The internal energy of the gas may change
- The gas may do some external work by expanding
According to the first law of Thermodynamics:
δQ = δW + ΔU
When a system executes a process, the change in stored energy of the system is numerically equal to the net heat interaction minus the network interaction during the process:
ΔU = δQ – δW where U is the internal energy which is introduced by this law.
For the cyclic process: ΔU = 0
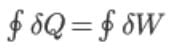
The first law of thermodynamics states that for a cyclic process, the cyclic integral of heat is equal to the cyclic integral of work.
The second law of thermodynamics introduces the concept of entropy.
Free Test
FREE
| Start Free Test |
Community Answer
Which of the following is incorrect regarding the first law of thermod...
The first law of thermodynamics, also known as the law of energy conservation, is a fundamental principle in thermodynamics that states that energy can neither be created nor destroyed, but it can be transferred from one form to another or converted from one system to another. In other words, the total energy of an isolated system remains constant.
a) Restatement of Conservation of Energy:
The first law of thermodynamics is indeed a restatement of the principle of conservation of energy, which is a fundamental law of physics. It simply states that energy cannot be created or destroyed, only transformed.
b) Applicable to Cyclic Processes:
The first law of thermodynamics is applicable to all processes, including cyclic processes. A cyclic process is one in which the system returns to its initial state after completing a full cycle. The first law of thermodynamics ensures that the total energy of the system remains unchanged throughout the cycle.
c) Introduction of Entropy:
The first law of thermodynamics does not introduce the concept of entropy. Entropy is a measure of the disorder or randomness of a system. The concept of entropy is introduced by the second law of thermodynamics, which states that the entropy of an isolated system tends to increase over time.
d) Introduction of Internal Energy:
The first law of thermodynamics introduces the concept of internal energy. Internal energy is the total energy contained within a system, including the kinetic energy of its particles and the potential energy associated with their interactions. The first law of thermodynamics states that the change in internal energy of a system is equal to the heat added to the system minus the work done by the system.
Therefore, the incorrect statement regarding the first law of thermodynamics is option C, which suggests that it introduces the concept of entropy. The concept of entropy is actually introduced by the second law of thermodynamics.
a) Restatement of Conservation of Energy:
The first law of thermodynamics is indeed a restatement of the principle of conservation of energy, which is a fundamental law of physics. It simply states that energy cannot be created or destroyed, only transformed.
b) Applicable to Cyclic Processes:
The first law of thermodynamics is applicable to all processes, including cyclic processes. A cyclic process is one in which the system returns to its initial state after completing a full cycle. The first law of thermodynamics ensures that the total energy of the system remains unchanged throughout the cycle.
c) Introduction of Entropy:
The first law of thermodynamics does not introduce the concept of entropy. Entropy is a measure of the disorder or randomness of a system. The concept of entropy is introduced by the second law of thermodynamics, which states that the entropy of an isolated system tends to increase over time.
d) Introduction of Internal Energy:
The first law of thermodynamics introduces the concept of internal energy. Internal energy is the total energy contained within a system, including the kinetic energy of its particles and the potential energy associated with their interactions. The first law of thermodynamics states that the change in internal energy of a system is equal to the heat added to the system minus the work done by the system.
Therefore, the incorrect statement regarding the first law of thermodynamics is option C, which suggests that it introduces the concept of entropy. The concept of entropy is actually introduced by the second law of thermodynamics.

|
Explore Courses for Railways exam
|

|
Similar Railways Doubts
Which of the following is incorrect regarding the first law of thermodynamics?a)It is a restatement of the principle of conservation of energyb)It is applicable to the cyclic processc)It introduces the concept of entropyd)It introduces the concept of internal energyCorrect answer is option 'C'. Can you explain this answer?
Question Description
Which of the following is incorrect regarding the first law of thermodynamics?a)It is a restatement of the principle of conservation of energyb)It is applicable to the cyclic processc)It introduces the concept of entropyd)It introduces the concept of internal energyCorrect answer is option 'C'. Can you explain this answer? for Railways 2025 is part of Railways preparation. The Question and answers have been prepared according to the Railways exam syllabus. Information about Which of the following is incorrect regarding the first law of thermodynamics?a)It is a restatement of the principle of conservation of energyb)It is applicable to the cyclic processc)It introduces the concept of entropyd)It introduces the concept of internal energyCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Railways 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following is incorrect regarding the first law of thermodynamics?a)It is a restatement of the principle of conservation of energyb)It is applicable to the cyclic processc)It introduces the concept of entropyd)It introduces the concept of internal energyCorrect answer is option 'C'. Can you explain this answer?.
Which of the following is incorrect regarding the first law of thermodynamics?a)It is a restatement of the principle of conservation of energyb)It is applicable to the cyclic processc)It introduces the concept of entropyd)It introduces the concept of internal energyCorrect answer is option 'C'. Can you explain this answer? for Railways 2025 is part of Railways preparation. The Question and answers have been prepared according to the Railways exam syllabus. Information about Which of the following is incorrect regarding the first law of thermodynamics?a)It is a restatement of the principle of conservation of energyb)It is applicable to the cyclic processc)It introduces the concept of entropyd)It introduces the concept of internal energyCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Railways 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following is incorrect regarding the first law of thermodynamics?a)It is a restatement of the principle of conservation of energyb)It is applicable to the cyclic processc)It introduces the concept of entropyd)It introduces the concept of internal energyCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Which of the following is incorrect regarding the first law of thermodynamics?a)It is a restatement of the principle of conservation of energyb)It is applicable to the cyclic processc)It introduces the concept of entropyd)It introduces the concept of internal energyCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Railways.
Download more important topics, notes, lectures and mock test series for Railways Exam by signing up for free.
Here you can find the meaning of Which of the following is incorrect regarding the first law of thermodynamics?a)It is a restatement of the principle of conservation of energyb)It is applicable to the cyclic processc)It introduces the concept of entropyd)It introduces the concept of internal energyCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following is incorrect regarding the first law of thermodynamics?a)It is a restatement of the principle of conservation of energyb)It is applicable to the cyclic processc)It introduces the concept of entropyd)It introduces the concept of internal energyCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Which of the following is incorrect regarding the first law of thermodynamics?a)It is a restatement of the principle of conservation of energyb)It is applicable to the cyclic processc)It introduces the concept of entropyd)It introduces the concept of internal energyCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Which of the following is incorrect regarding the first law of thermodynamics?a)It is a restatement of the principle of conservation of energyb)It is applicable to the cyclic processc)It introduces the concept of entropyd)It introduces the concept of internal energyCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following is incorrect regarding the first law of thermodynamics?a)It is a restatement of the principle of conservation of energyb)It is applicable to the cyclic processc)It introduces the concept of entropyd)It introduces the concept of internal energyCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Railways tests.

|
Explore Courses for Railways exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























