Railways Exam > Railways Questions > The internal energy of a gas obeying van der ...
Start Learning for Free
The internal energy of a gas obeying van der Waals, equation (p + a/V2)(V - b) = RT depends on its
- a)Temperature
- b)temperature and pressure
- c)temperature and specific volume
- d)pressure and specific volume
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
The internal energy of a gas obeying van der Waals, equation (p + a/V2...
internal energy: U = u (T, V)
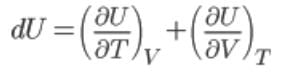
For a real gas, the internal energy if a function of both the temperature and the specific volume.
That is,
U = f(T,u)
For ideal gas (∂U/∂V)T = 0
∴ U = U (T) only
Important Points
For an ideal gas (no intermolecular interactions and no molecular volume), appropriate equation of state would be: PV = nRT ⇒ V = nRT/P i.e. V = f(T,P,n)
There are many equations of state describing real gases. These equations take in consideration molecular volume and interactions. The most well-known such equation is the Van der Waals equation.
The internal energy of an ideal gas is a function of temperature only and is independent of pressure and volume. u = u(T)
Most Upvoted Answer
The internal energy of a gas obeying van der Waals, equation (p + a/V2...
Internal Energy of a Gas obeying van der Waals Equation
The van der Waals equation of state is an improvement over the ideal gas equation by considering the intermolecular forces and the finite size of gas molecules. It is given by:
(p + a/V^2)(V - b) = RT
where:
p is the pressure of the gas
V is the volume of the gas
a and b are constants related to the attractive and repulsive forces between molecules
R is the ideal gas constant
T is the temperature of the gas
Factors affecting the Internal Energy
The internal energy of a gas is the sum of the kinetic and potential energies of its molecules. In the case of a gas obeying the van der Waals equation, the internal energy depends on the temperature and specific volume.
Temperature
Temperature is a measure of the average kinetic energy of the gas molecules. As the temperature increases, the kinetic energy of the molecules also increases. This leads to an increase in the internal energy of the gas.
Specific Volume
Specific volume is the volume occupied by a unit mass of the gas. In the van der Waals equation, the term (V - b) represents the effective volume available for the gas molecules to move around. The constant b accounts for the volume occupied by the gas molecules themselves.
As the specific volume decreases (i.e., the gas becomes more compressed), the available volume for the gas molecules to move decreases. This results in an increase in the potential energy of the gas molecules and hence an increase in the internal energy.
Conclusion
In conclusion, the internal energy of a gas obeying the van der Waals equation depends on both the temperature and specific volume. The temperature affects the kinetic energy of the gas molecules, while the specific volume affects the potential energy of the gas molecules. Both of these factors contribute to the overall internal energy of the gas.
The van der Waals equation of state is an improvement over the ideal gas equation by considering the intermolecular forces and the finite size of gas molecules. It is given by:
(p + a/V^2)(V - b) = RT
where:
p is the pressure of the gas
V is the volume of the gas
a and b are constants related to the attractive and repulsive forces between molecules
R is the ideal gas constant
T is the temperature of the gas
Factors affecting the Internal Energy
The internal energy of a gas is the sum of the kinetic and potential energies of its molecules. In the case of a gas obeying the van der Waals equation, the internal energy depends on the temperature and specific volume.
Temperature
Temperature is a measure of the average kinetic energy of the gas molecules. As the temperature increases, the kinetic energy of the molecules also increases. This leads to an increase in the internal energy of the gas.
Specific Volume
Specific volume is the volume occupied by a unit mass of the gas. In the van der Waals equation, the term (V - b) represents the effective volume available for the gas molecules to move around. The constant b accounts for the volume occupied by the gas molecules themselves.
As the specific volume decreases (i.e., the gas becomes more compressed), the available volume for the gas molecules to move decreases. This results in an increase in the potential energy of the gas molecules and hence an increase in the internal energy.
Conclusion
In conclusion, the internal energy of a gas obeying the van der Waals equation depends on both the temperature and specific volume. The temperature affects the kinetic energy of the gas molecules, while the specific volume affects the potential energy of the gas molecules. Both of these factors contribute to the overall internal energy of the gas.
Attention Railways Students!
To make sure you are not studying endlessly, EduRev has designed Railways study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Railways.

|
Explore Courses for Railways exam
|

|
Similar Railways Doubts
The internal energy of a gas obeying van der Waals, equation (p + a/V2)(V - b) = RT depends on itsa)Temperatureb)temperature and pressurec)temperature and specific volumed)pressure and specific volumeCorrect answer is option 'C'. Can you explain this answer?
Question Description
The internal energy of a gas obeying van der Waals, equation (p + a/V2)(V - b) = RT depends on itsa)Temperatureb)temperature and pressurec)temperature and specific volumed)pressure and specific volumeCorrect answer is option 'C'. Can you explain this answer? for Railways 2024 is part of Railways preparation. The Question and answers have been prepared according to the Railways exam syllabus. Information about The internal energy of a gas obeying van der Waals, equation (p + a/V2)(V - b) = RT depends on itsa)Temperatureb)temperature and pressurec)temperature and specific volumed)pressure and specific volumeCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Railways 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The internal energy of a gas obeying van der Waals, equation (p + a/V2)(V - b) = RT depends on itsa)Temperatureb)temperature and pressurec)temperature and specific volumed)pressure and specific volumeCorrect answer is option 'C'. Can you explain this answer?.
The internal energy of a gas obeying van der Waals, equation (p + a/V2)(V - b) = RT depends on itsa)Temperatureb)temperature and pressurec)temperature and specific volumed)pressure and specific volumeCorrect answer is option 'C'. Can you explain this answer? for Railways 2024 is part of Railways preparation. The Question and answers have been prepared according to the Railways exam syllabus. Information about The internal energy of a gas obeying van der Waals, equation (p + a/V2)(V - b) = RT depends on itsa)Temperatureb)temperature and pressurec)temperature and specific volumed)pressure and specific volumeCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for Railways 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The internal energy of a gas obeying van der Waals, equation (p + a/V2)(V - b) = RT depends on itsa)Temperatureb)temperature and pressurec)temperature and specific volumed)pressure and specific volumeCorrect answer is option 'C'. Can you explain this answer?.
Solutions for The internal energy of a gas obeying van der Waals, equation (p + a/V2)(V - b) = RT depends on itsa)Temperatureb)temperature and pressurec)temperature and specific volumed)pressure and specific volumeCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for Railways.
Download more important topics, notes, lectures and mock test series for Railways Exam by signing up for free.
Here you can find the meaning of The internal energy of a gas obeying van der Waals, equation (p + a/V2)(V - b) = RT depends on itsa)Temperatureb)temperature and pressurec)temperature and specific volumed)pressure and specific volumeCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The internal energy of a gas obeying van der Waals, equation (p + a/V2)(V - b) = RT depends on itsa)Temperatureb)temperature and pressurec)temperature and specific volumed)pressure and specific volumeCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for The internal energy of a gas obeying van der Waals, equation (p + a/V2)(V - b) = RT depends on itsa)Temperatureb)temperature and pressurec)temperature and specific volumed)pressure and specific volumeCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of The internal energy of a gas obeying van der Waals, equation (p + a/V2)(V - b) = RT depends on itsa)Temperatureb)temperature and pressurec)temperature and specific volumed)pressure and specific volumeCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The internal energy of a gas obeying van der Waals, equation (p + a/V2)(V - b) = RT depends on itsa)Temperatureb)temperature and pressurec)temperature and specific volumed)pressure and specific volumeCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice Railways tests.

|
Explore Courses for Railways exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























