Chemistry Exam > Chemistry Questions > At very low concentrations of azomethane, its...
Start Learning for Free
At very low concentrations of azomethane, its decomposition follows which order?
- a)First order
- b)Zero order
- c)Third order
- d)Second order
Correct answer is option 'D'. Can you explain this answer?
Most Upvoted Answer
At very low concentrations of azomethane, its decomposition follows wh...
Decomposition of Azomethane
Azomethane (CH3-N=N-CH3), also known as dimethyl diazene, is an organic compound commonly used as a reagent in organic chemistry. At very low concentrations, the decomposition of azomethane follows second order kinetics.
Explanation:
Second Order Reaction
A second order reaction is characterized by a rate equation that is proportional to the square of the concentration of the reactant(s). In the case of azomethane decomposition, the rate equation can be represented as:
Rate = k[Azomethane]^2
where [Azomethane] represents the concentration of azomethane and k is the rate constant.
Reasoning:
The decomposition of azomethane involves the breaking of the N=N bond, resulting in the formation of two methyl radicals. Since the reaction involves the collision of two azomethane molecules, the rate of the reaction is proportional to the product of their concentrations, [Azomethane]^2, making it a second order reaction.
Experimental Evidence:
Experimental studies have confirmed that the decomposition of azomethane follows second order kinetics. By monitoring the change in azomethane concentration over time, the rate constant can be determined and the reaction order can be established.
Importance of Reaction Order:
Determining the reaction order is crucial for understanding the kinetics of a reaction. It allows us to predict how changes in reactant concentrations will affect the reaction rate. In the case of azomethane, knowing that it follows second order kinetics can help in designing appropriate reaction conditions and optimizing reaction parameters.
In conclusion, the decomposition of azomethane at very low concentrations follows second order kinetics. This is evidenced by the rate equation, experimental studies, and the nature of the reaction mechanism.
Azomethane (CH3-N=N-CH3), also known as dimethyl diazene, is an organic compound commonly used as a reagent in organic chemistry. At very low concentrations, the decomposition of azomethane follows second order kinetics.
Explanation:
Second Order Reaction
A second order reaction is characterized by a rate equation that is proportional to the square of the concentration of the reactant(s). In the case of azomethane decomposition, the rate equation can be represented as:
Rate = k[Azomethane]^2
where [Azomethane] represents the concentration of azomethane and k is the rate constant.
Reasoning:
The decomposition of azomethane involves the breaking of the N=N bond, resulting in the formation of two methyl radicals. Since the reaction involves the collision of two azomethane molecules, the rate of the reaction is proportional to the product of their concentrations, [Azomethane]^2, making it a second order reaction.
Experimental Evidence:
Experimental studies have confirmed that the decomposition of azomethane follows second order kinetics. By monitoring the change in azomethane concentration over time, the rate constant can be determined and the reaction order can be established.
Importance of Reaction Order:
Determining the reaction order is crucial for understanding the kinetics of a reaction. It allows us to predict how changes in reactant concentrations will affect the reaction rate. In the case of azomethane, knowing that it follows second order kinetics can help in designing appropriate reaction conditions and optimizing reaction parameters.
In conclusion, the decomposition of azomethane at very low concentrations follows second order kinetics. This is evidenced by the rate equation, experimental studies, and the nature of the reaction mechanism.
Free Test
FREE
| Start Free Test |
Community Answer
At very low concentrations of azomethane, its decomposition follows wh...
The rate expression for decomposition of azomethane is:
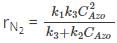
Where, k1, k2 and k3 are rate constants of the 3 intermediate reactions involved in azomethane formation.
At low concentration, k2 CAzo << k3
Hence, rN2=k1CAzo2.
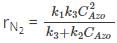
Where, k1, k2 and k3 are rate constants of the 3 intermediate reactions involved in azomethane formation.
At low concentration, k2 CAzo << k3
Hence, rN2=k1CAzo2.

|
Explore Courses for Chemistry exam
|

|
Question Description
At very low concentrations of azomethane, its decomposition follows which order?a)First orderb)Zero orderc)Third orderd)Second orderCorrect answer is option 'D'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about At very low concentrations of azomethane, its decomposition follows which order?a)First orderb)Zero orderc)Third orderd)Second orderCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for At very low concentrations of azomethane, its decomposition follows which order?a)First orderb)Zero orderc)Third orderd)Second orderCorrect answer is option 'D'. Can you explain this answer?.
At very low concentrations of azomethane, its decomposition follows which order?a)First orderb)Zero orderc)Third orderd)Second orderCorrect answer is option 'D'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about At very low concentrations of azomethane, its decomposition follows which order?a)First orderb)Zero orderc)Third orderd)Second orderCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for At very low concentrations of azomethane, its decomposition follows which order?a)First orderb)Zero orderc)Third orderd)Second orderCorrect answer is option 'D'. Can you explain this answer?.
Solutions for At very low concentrations of azomethane, its decomposition follows which order?a)First orderb)Zero orderc)Third orderd)Second orderCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of At very low concentrations of azomethane, its decomposition follows which order?a)First orderb)Zero orderc)Third orderd)Second orderCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
At very low concentrations of azomethane, its decomposition follows which order?a)First orderb)Zero orderc)Third orderd)Second orderCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for At very low concentrations of azomethane, its decomposition follows which order?a)First orderb)Zero orderc)Third orderd)Second orderCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of At very low concentrations of azomethane, its decomposition follows which order?a)First orderb)Zero orderc)Third orderd)Second orderCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice At very low concentrations of azomethane, its decomposition follows which order?a)First orderb)Zero orderc)Third orderd)Second orderCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















