Chemistry Exam > Chemistry Questions > Which of the following compounds does not giv...
Start Learning for Free
Which of the following compounds does not give a tertiary alcohol upon reaction with methylmagnesium bromide/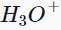 ?
?
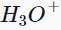 ?
?- a)4-heptanone
- b)Ethyl benzoate
- c)4,4-dimethylcyclohexanone
- d)3-methylpentanal
Correct answer is option 'D'. Can you explain this answer?
Most Upvoted Answer
Which of the following compounds does not give a tertiary alcohol upon...
Reaction of Methylmagnesium bromide with Carbonyl Compounds
Carbonyl compounds contain a carbonyl group (C=O) and can undergo nucleophilic addition reactions with nucleophiles such as methylmagnesium bromide (CH3MgBr) to form alcohols. The reaction proceeds through the formation of an unstable intermediate called an alkoxy magnesium halide, which is then hydrolyzed to form the alcohol.
Reaction of Methylmagnesium bromide with 4-Heptanone
4-heptanone is a ketone with the formula C7H14O. When it is treated with methylmagnesium bromide, a Grignard reagent, it undergoes a nucleophilic addition reaction to form an alkoxy magnesium halide intermediate. The intermediate then undergoes hydrolysis to form the corresponding alcohol.
C7H14O + CH3MgBr → C7H13OMgBr + CH4
C7H13OMgBr + H2O → C7H16O + MgBrOH
The alcohol formed is a tertiary alcohol, 2,2,3,3-tetramethylbutan-2-ol.
Reaction of Methylmagnesium bromide with 3-Methylpentanal
3-methylpentanal is an aldehyde with the formula C6H12O. When it is treated with methylmagnesium bromide, it undergoes a nucleophilic addition reaction to form an alkoxy magnesium halide intermediate. The intermediate then undergoes hydrolysis to form the corresponding alcohol.
C6H12O + CH3MgBr → C6H11OMgBr + CH4
C6H11OMgBr + H2O → C6H14O + MgBrOH
The alcohol formed is a tertiary alcohol, 2,2-dimethyl-3-pentanol.
Reaction of Methylmagnesium bromide with Ethyl benzoate
Ethyl benzoate is an ester with the formula C9H10O2. When it is treated with methylmagnesium bromide, it undergoes a nucleophilic addition reaction to form an alkoxy magnesium halide intermediate. The intermediate then undergoes hydrolysis to form the corresponding alcohol.
C9H10O2 + CH3MgBr → C9H9OMgBr + C2H5Br
C9H9OMgBr + H2O → C9H12O2 + MgBrOH
The alcohol formed is a tertiary alcohol, 2-phenylethanol.
Reaction of Methylmagnesium bromide with 4,4-Dimethylcyclohexanone
4,4-dimethylcyclohexanone is a ketone with the formula C10H18O. When it is treated with methylmagnesium bromide, it undergoes a nucleophilic addition reaction to form an alkoxy magnesium halide intermediate. The intermediate then undergoes hydrolysis to form the corresponding alcohol.
C10H18O + CH3MgBr → C10H17OMgBr + CH4
C10H17OMgBr + H2O → C10H20O + MgBrOH
The alcohol formed is a tertiary alcohol, 4
Carbonyl compounds contain a carbonyl group (C=O) and can undergo nucleophilic addition reactions with nucleophiles such as methylmagnesium bromide (CH3MgBr) to form alcohols. The reaction proceeds through the formation of an unstable intermediate called an alkoxy magnesium halide, which is then hydrolyzed to form the alcohol.
Reaction of Methylmagnesium bromide with 4-Heptanone
4-heptanone is a ketone with the formula C7H14O. When it is treated with methylmagnesium bromide, a Grignard reagent, it undergoes a nucleophilic addition reaction to form an alkoxy magnesium halide intermediate. The intermediate then undergoes hydrolysis to form the corresponding alcohol.
C7H14O + CH3MgBr → C7H13OMgBr + CH4
C7H13OMgBr + H2O → C7H16O + MgBrOH
The alcohol formed is a tertiary alcohol, 2,2,3,3-tetramethylbutan-2-ol.
Reaction of Methylmagnesium bromide with 3-Methylpentanal
3-methylpentanal is an aldehyde with the formula C6H12O. When it is treated with methylmagnesium bromide, it undergoes a nucleophilic addition reaction to form an alkoxy magnesium halide intermediate. The intermediate then undergoes hydrolysis to form the corresponding alcohol.
C6H12O + CH3MgBr → C6H11OMgBr + CH4
C6H11OMgBr + H2O → C6H14O + MgBrOH
The alcohol formed is a tertiary alcohol, 2,2-dimethyl-3-pentanol.
Reaction of Methylmagnesium bromide with Ethyl benzoate
Ethyl benzoate is an ester with the formula C9H10O2. When it is treated with methylmagnesium bromide, it undergoes a nucleophilic addition reaction to form an alkoxy magnesium halide intermediate. The intermediate then undergoes hydrolysis to form the corresponding alcohol.
C9H10O2 + CH3MgBr → C9H9OMgBr + C2H5Br
C9H9OMgBr + H2O → C9H12O2 + MgBrOH
The alcohol formed is a tertiary alcohol, 2-phenylethanol.
Reaction of Methylmagnesium bromide with 4,4-Dimethylcyclohexanone
4,4-dimethylcyclohexanone is a ketone with the formula C10H18O. When it is treated with methylmagnesium bromide, it undergoes a nucleophilic addition reaction to form an alkoxy magnesium halide intermediate. The intermediate then undergoes hydrolysis to form the corresponding alcohol.
C10H18O + CH3MgBr → C10H17OMgBr + CH4
C10H17OMgBr + H2O → C10H20O + MgBrOH
The alcohol formed is a tertiary alcohol, 4
Free Test
FREE
| Start Free Test |
Community Answer
Which of the following compounds does not give a tertiary alcohol upon...
4-heptanone does not give a tertiary alcohol upon reaction with methylmagnesium bromide, as the Grignard Reaction is the addition of an organomagnesium halide (Grignard reagent) to a ketone or aldehyde, to form a tertiary or secondary alcohol, respectively.

|
Explore Courses for Chemistry exam
|

|
Question Description
Which of the following compounds does not give a tertiary alcohol upon reaction with methylmagnesium bromide/?a)4-heptanoneb)Ethyl benzoatec)4,4-dimethylcyclohexanoned)3-methylpentanalCorrect answer is option 'D'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Which of the following compounds does not give a tertiary alcohol upon reaction with methylmagnesium bromide/?a)4-heptanoneb)Ethyl benzoatec)4,4-dimethylcyclohexanoned)3-methylpentanalCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following compounds does not give a tertiary alcohol upon reaction with methylmagnesium bromide/?a)4-heptanoneb)Ethyl benzoatec)4,4-dimethylcyclohexanoned)3-methylpentanalCorrect answer is option 'D'. Can you explain this answer?.
Which of the following compounds does not give a tertiary alcohol upon reaction with methylmagnesium bromide/?a)4-heptanoneb)Ethyl benzoatec)4,4-dimethylcyclohexanoned)3-methylpentanalCorrect answer is option 'D'. Can you explain this answer? for Chemistry 2025 is part of Chemistry preparation. The Question and answers have been prepared according to the Chemistry exam syllabus. Information about Which of the following compounds does not give a tertiary alcohol upon reaction with methylmagnesium bromide/?a)4-heptanoneb)Ethyl benzoatec)4,4-dimethylcyclohexanoned)3-methylpentanalCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Chemistry 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following compounds does not give a tertiary alcohol upon reaction with methylmagnesium bromide/?a)4-heptanoneb)Ethyl benzoatec)4,4-dimethylcyclohexanoned)3-methylpentanalCorrect answer is option 'D'. Can you explain this answer?.
Solutions for Which of the following compounds does not give a tertiary alcohol upon reaction with methylmagnesium bromide/?a)4-heptanoneb)Ethyl benzoatec)4,4-dimethylcyclohexanoned)3-methylpentanalCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Chemistry.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Here you can find the meaning of Which of the following compounds does not give a tertiary alcohol upon reaction with methylmagnesium bromide/?a)4-heptanoneb)Ethyl benzoatec)4,4-dimethylcyclohexanoned)3-methylpentanalCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following compounds does not give a tertiary alcohol upon reaction with methylmagnesium bromide/?a)4-heptanoneb)Ethyl benzoatec)4,4-dimethylcyclohexanoned)3-methylpentanalCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for Which of the following compounds does not give a tertiary alcohol upon reaction with methylmagnesium bromide/?a)4-heptanoneb)Ethyl benzoatec)4,4-dimethylcyclohexanoned)3-methylpentanalCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of Which of the following compounds does not give a tertiary alcohol upon reaction with methylmagnesium bromide/?a)4-heptanoneb)Ethyl benzoatec)4,4-dimethylcyclohexanoned)3-methylpentanalCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following compounds does not give a tertiary alcohol upon reaction with methylmagnesium bromide/?a)4-heptanoneb)Ethyl benzoatec)4,4-dimethylcyclohexanoned)3-methylpentanalCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Chemistry tests.

|
Explore Courses for Chemistry exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























