JEE Exam > JEE Questions > Directions: The following question has four ...
Start Learning for Free
Directions: The following question has four choices, out of which one or more is/are correct.
Select correct statement(s) about the chemical behaviour of oxo ions of halogens.
- a)ClO2 and Cl2O are used as bleaching agents for paper pulp and textiles.
- b)OCl- disproportionates in alkaline medium.
- c)BrO3- liberates Br2 with iodine in acidic medium.
- d)HClO2 liberates ICl on reaction with KI and HCl.
Correct answer is option 'A,B,C'. Can you explain this answer?
Most Upvoted Answer
Directions: The following question has four choices, out of which one...
Both chlorine monoxide (Cl2O) as well as chlorine dioxide (ClO2) are used as bleaching agents to bleach wood pulp as well as to disinfect drinking water.
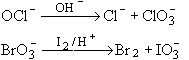
Hence, options (a), (b) and (c) are correct.
However, the reaction of chlorous acid with KI and HCl proceeds as follows.
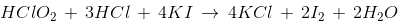
Free Test
FREE
| Start Free Test |
Community Answer
Directions: The following question has four choices, out of which one...
Chemical Behaviour of Oxo Ions of Halogens
a) ClO2 and Cl2O are used as bleaching agents for paper pulp and textiles.
- ClO2, also known as chlorine dioxide, is a powerful bleaching agent commonly used in the paper and textile industries. It has strong oxidizing properties and can effectively remove colorants and stains from materials.
- Cl2O, also known as chlorine monoxide, is another bleaching agent used in the same industries. It reacts with water to form hypochlorous acid (HClO), which further acts as a bleaching agent.
b) OCl- disproportionates in alkaline medium.
- Disproportionation refers to the reaction of a substance in which it simultaneously undergoes both oxidation and reduction. In the case of OCl-, it can disproportionates in an alkaline medium, forming both chloride ions (Cl-) and perchlorate ions (ClO4-).
- The reaction can be represented as: 3OCl- + 6OH- → 5Cl- + ClO3- + 3H2O
c) BrO3- liberates Br2 with iodine in acidic medium.
- BrO3-, also known as bromate ion, can liberate bromine (Br2) when reacted with iodine (I2) in an acidic medium.
- The reaction can be represented as: 5BrO3- + 6I- + 6H+ → 3Br2 + 6H2O + 3I3-
d) HClO2 liberates ICl on reaction with KI and HCl.
- HClO2, also known as chlorous acid, can liberate iodine monochloride (ICl) when reacted with potassium iodide (KI) and hydrochloric acid (HCl).
- The reaction can be represented as: 2HClO2 + 4KI + 2HCl → 4KCl + ICl + 3H2O
Therefore, the correct statements about the chemical behaviour of oxo ions of halogens are A (ClO2 and Cl2O are used as bleaching agents for paper pulp and textiles), B (OCl- disproportionates in an alkaline medium), and C (BrO3- liberates Br2 with iodine in an acidic medium).
a) ClO2 and Cl2O are used as bleaching agents for paper pulp and textiles.
- ClO2, also known as chlorine dioxide, is a powerful bleaching agent commonly used in the paper and textile industries. It has strong oxidizing properties and can effectively remove colorants and stains from materials.
- Cl2O, also known as chlorine monoxide, is another bleaching agent used in the same industries. It reacts with water to form hypochlorous acid (HClO), which further acts as a bleaching agent.
b) OCl- disproportionates in alkaline medium.
- Disproportionation refers to the reaction of a substance in which it simultaneously undergoes both oxidation and reduction. In the case of OCl-, it can disproportionates in an alkaline medium, forming both chloride ions (Cl-) and perchlorate ions (ClO4-).
- The reaction can be represented as: 3OCl- + 6OH- → 5Cl- + ClO3- + 3H2O
c) BrO3- liberates Br2 with iodine in acidic medium.
- BrO3-, also known as bromate ion, can liberate bromine (Br2) when reacted with iodine (I2) in an acidic medium.
- The reaction can be represented as: 5BrO3- + 6I- + 6H+ → 3Br2 + 6H2O + 3I3-
d) HClO2 liberates ICl on reaction with KI and HCl.
- HClO2, also known as chlorous acid, can liberate iodine monochloride (ICl) when reacted with potassium iodide (KI) and hydrochloric acid (HCl).
- The reaction can be represented as: 2HClO2 + 4KI + 2HCl → 4KCl + ICl + 3H2O
Therefore, the correct statements about the chemical behaviour of oxo ions of halogens are A (ClO2 and Cl2O are used as bleaching agents for paper pulp and textiles), B (OCl- disproportionates in an alkaline medium), and C (BrO3- liberates Br2 with iodine in an acidic medium).

|
Explore Courses for JEE exam
|

|
Directions: The following question has four choices, out of which one or more is/are correct.Select correct statement(s) about the chemical behaviour of oxo ions of halogens.a)ClO2 and Cl2O are used as bleaching agents for paper pulp and textiles.b)OCl- disproportionates in alkaline medium.c)BrO3- liberates Br2 with iodine in acidic medium.d)HClO2 liberates ICl on reaction with KI and HCl.Correct answer is option 'A,B,C'. Can you explain this answer?
Question Description
Directions: The following question has four choices, out of which one or more is/are correct.Select correct statement(s) about the chemical behaviour of oxo ions of halogens.a)ClO2 and Cl2O are used as bleaching agents for paper pulp and textiles.b)OCl- disproportionates in alkaline medium.c)BrO3- liberates Br2 with iodine in acidic medium.d)HClO2 liberates ICl on reaction with KI and HCl.Correct answer is option 'A,B,C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Directions: The following question has four choices, out of which one or more is/are correct.Select correct statement(s) about the chemical behaviour of oxo ions of halogens.a)ClO2 and Cl2O are used as bleaching agents for paper pulp and textiles.b)OCl- disproportionates in alkaline medium.c)BrO3- liberates Br2 with iodine in acidic medium.d)HClO2 liberates ICl on reaction with KI and HCl.Correct answer is option 'A,B,C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions: The following question has four choices, out of which one or more is/are correct.Select correct statement(s) about the chemical behaviour of oxo ions of halogens.a)ClO2 and Cl2O are used as bleaching agents for paper pulp and textiles.b)OCl- disproportionates in alkaline medium.c)BrO3- liberates Br2 with iodine in acidic medium.d)HClO2 liberates ICl on reaction with KI and HCl.Correct answer is option 'A,B,C'. Can you explain this answer?.
Directions: The following question has four choices, out of which one or more is/are correct.Select correct statement(s) about the chemical behaviour of oxo ions of halogens.a)ClO2 and Cl2O are used as bleaching agents for paper pulp and textiles.b)OCl- disproportionates in alkaline medium.c)BrO3- liberates Br2 with iodine in acidic medium.d)HClO2 liberates ICl on reaction with KI and HCl.Correct answer is option 'A,B,C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Directions: The following question has four choices, out of which one or more is/are correct.Select correct statement(s) about the chemical behaviour of oxo ions of halogens.a)ClO2 and Cl2O are used as bleaching agents for paper pulp and textiles.b)OCl- disproportionates in alkaline medium.c)BrO3- liberates Br2 with iodine in acidic medium.d)HClO2 liberates ICl on reaction with KI and HCl.Correct answer is option 'A,B,C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions: The following question has four choices, out of which one or more is/are correct.Select correct statement(s) about the chemical behaviour of oxo ions of halogens.a)ClO2 and Cl2O are used as bleaching agents for paper pulp and textiles.b)OCl- disproportionates in alkaline medium.c)BrO3- liberates Br2 with iodine in acidic medium.d)HClO2 liberates ICl on reaction with KI and HCl.Correct answer is option 'A,B,C'. Can you explain this answer?.
Solutions for Directions: The following question has four choices, out of which one or more is/are correct.Select correct statement(s) about the chemical behaviour of oxo ions of halogens.a)ClO2 and Cl2O are used as bleaching agents for paper pulp and textiles.b)OCl- disproportionates in alkaline medium.c)BrO3- liberates Br2 with iodine in acidic medium.d)HClO2 liberates ICl on reaction with KI and HCl.Correct answer is option 'A,B,C'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Directions: The following question has four choices, out of which one or more is/are correct.Select correct statement(s) about the chemical behaviour of oxo ions of halogens.a)ClO2 and Cl2O are used as bleaching agents for paper pulp and textiles.b)OCl- disproportionates in alkaline medium.c)BrO3- liberates Br2 with iodine in acidic medium.d)HClO2 liberates ICl on reaction with KI and HCl.Correct answer is option 'A,B,C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Directions: The following question has four choices, out of which one or more is/are correct.Select correct statement(s) about the chemical behaviour of oxo ions of halogens.a)ClO2 and Cl2O are used as bleaching agents for paper pulp and textiles.b)OCl- disproportionates in alkaline medium.c)BrO3- liberates Br2 with iodine in acidic medium.d)HClO2 liberates ICl on reaction with KI and HCl.Correct answer is option 'A,B,C'. Can you explain this answer?, a detailed solution for Directions: The following question has four choices, out of which one or more is/are correct.Select correct statement(s) about the chemical behaviour of oxo ions of halogens.a)ClO2 and Cl2O are used as bleaching agents for paper pulp and textiles.b)OCl- disproportionates in alkaline medium.c)BrO3- liberates Br2 with iodine in acidic medium.d)HClO2 liberates ICl on reaction with KI and HCl.Correct answer is option 'A,B,C'. Can you explain this answer? has been provided alongside types of Directions: The following question has four choices, out of which one or more is/are correct.Select correct statement(s) about the chemical behaviour of oxo ions of halogens.a)ClO2 and Cl2O are used as bleaching agents for paper pulp and textiles.b)OCl- disproportionates in alkaline medium.c)BrO3- liberates Br2 with iodine in acidic medium.d)HClO2 liberates ICl on reaction with KI and HCl.Correct answer is option 'A,B,C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Directions: The following question has four choices, out of which one or more is/are correct.Select correct statement(s) about the chemical behaviour of oxo ions of halogens.a)ClO2 and Cl2O are used as bleaching agents for paper pulp and textiles.b)OCl- disproportionates in alkaline medium.c)BrO3- liberates Br2 with iodine in acidic medium.d)HClO2 liberates ICl on reaction with KI and HCl.Correct answer is option 'A,B,C'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























