JEE Exam > JEE Questions > Valence shell electron-pair repulsion theory...
Start Learning for Free
Valence shell electron-pair repulsion theory predicts the shape of a molecule by considering the most stable configuration of the bond angles in the molecule. The main points of the theory are
(i) Electron pairs in the valence shell of central atom of a molecule, whether bonding or lone pairs are regarded as occupying localised orbitals. These orbitals arrange themselves so as to minimize the mutual electronic repulsions.
(ii) The magnitude of the different types of electronic repulsions follow the order given below:
Lone pair-lone pair> lone pair-bonding pair > bonding pair-bonding pair. These repulsive forces alter the bond angles of the molecule or ion.
(iii) The electron repulsion between two pairs of electrons will be minimum if they stay as far as possible. On this basis, several studied. have been done
Which of the following statements is correct ?
- a)The bond angle of NCl3 is greater than NH3 as Pπ − dπ back-bonding is present in NCl3.
- b)BF3 is more acidic than BCl3 due to presence of Pπ − dπ back bonding in BF3
- c)When the bond multiplicity is increased, bond angle is decreased.
- d)The bond angle of SiCl4 is greater than CCl4
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
Valence shell electron-pair repulsion theory predicts the shape of a ...
Explanation:
NCl3 vs NH3 bond angle:
- In NCl3, the central nitrogen atom has a lone pair of electrons and three bond pairs. Due to the presence of the lone pair, there is greater repulsion among the electron pairs, leading to a smaller bond angle compared to NH3.
- In NH3, nitrogen has a lone pair and three bond pairs, resulting in a larger bond angle compared to NCl3.
Therefore, the statement "The bond angle of NCl3 is greater than NH3 as Pπ − dπ back-bonding is present in NCl3" is correct.
BF3 vs BCl3 acidity:
- BF3 is not acidic as it does not have any H+ ions to donate, whereas BCl3 can act as a Lewis acid by accepting a lone pair of electrons.
- The presence of Pπ − dπ back-bonding in BF3 does not make it more acidic compared to BCl3.
Therefore, the statement "BF3 is more acidic than BCl3 due to the presence of Pπ − dπ back-bonding in BF3" is incorrect.
Bond multiplicity and bond angle:
- When the bond multiplicity is increased, the bond angle is not necessarily decreased. The bond angle depends on the repulsion between electron pairs and the geometry of the molecule.
SiCl4 vs CCl4 bond angle:
- In SiCl4 and CCl4, both central atoms have four bond pairs and no lone pairs. Since both molecules have the same geometry (tetrahedral), the bond angle in SiCl4 is greater than that in CCl4 due to the larger size of the silicon atom compared to carbon.
Therefore, the statement "The bond angle of SiCl4 is greater than CCl4" is correct.
NCl3 vs NH3 bond angle:
- In NCl3, the central nitrogen atom has a lone pair of electrons and three bond pairs. Due to the presence of the lone pair, there is greater repulsion among the electron pairs, leading to a smaller bond angle compared to NH3.
- In NH3, nitrogen has a lone pair and three bond pairs, resulting in a larger bond angle compared to NCl3.
Therefore, the statement "The bond angle of NCl3 is greater than NH3 as Pπ − dπ back-bonding is present in NCl3" is correct.
BF3 vs BCl3 acidity:
- BF3 is not acidic as it does not have any H+ ions to donate, whereas BCl3 can act as a Lewis acid by accepting a lone pair of electrons.
- The presence of Pπ − dπ back-bonding in BF3 does not make it more acidic compared to BCl3.
Therefore, the statement "BF3 is more acidic than BCl3 due to the presence of Pπ − dπ back-bonding in BF3" is incorrect.
Bond multiplicity and bond angle:
- When the bond multiplicity is increased, the bond angle is not necessarily decreased. The bond angle depends on the repulsion between electron pairs and the geometry of the molecule.
SiCl4 vs CCl4 bond angle:
- In SiCl4 and CCl4, both central atoms have four bond pairs and no lone pairs. Since both molecules have the same geometry (tetrahedral), the bond angle in SiCl4 is greater than that in CCl4 due to the larger size of the silicon atom compared to carbon.
Therefore, the statement "The bond angle of SiCl4 is greater than CCl4" is correct.
Free Test
FREE
| Start Free Test |
Community Answer
Valence shell electron-pair repulsion theory predicts the shape of a ...
This problem includes the concept of bond angle the basis of Pπ − dπ back bonding and bond stifness, read all statements carefully and answer according to question using above concept.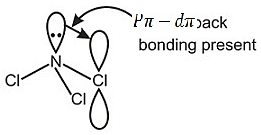

Due to presence of Pπ − dπ back bonding, NCl3 is sp2 hybridised but due to the absence of vacant d orbital in NH3 it can't form Pπ − dπ back bonding. So bond anlge of NCl3 is greater than NH3. Due to Pπ − dπ back bonding (d-orbitals available in Cl). The lone pair on N not available for exerting repulsion and hence bond angle in NCl3 > NH3.
In BF3 there is Pπ − dπ back bonding from filled orbitals of F creating a partial double bond character making it a poor Lewis acid than BCl3.
Bond multiplicity leads to more repulsion - double bond - lone pair repulsion is too high and hence bond angle increases.
SiCl4 and CCl4 bond angles are both tetrahedral (same).
Attention JEE Students!
To make sure you are not studying endlessly, EduRev has designed JEE study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in JEE.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Valence shell electron-pair repulsion theory predicts the shape of a molecule by considering the most stable configuration of the bond angles in the molecule. The main points of the theory are(i) Electron pairs in the valence shell of central atom of a molecule, whether bonding or lone pairs are regarded as occupying localised orbitals. These orbitals arrange themselves so as to minimize the mutual electronic repulsions.(ii) The magnitude of the different types of electronic repulsions follow the order given below:Lone pair-lone pair> lone pair-bonding pair > bonding pair-bonding pair. These repulsive forces alter the bond angles of the molecule or ion.(iii) The electron repulsion between two pairs of electrons will be minimum if they stay as far as possible. On this basis, several studied. have been doneWhich of the following statements is correct ?a)The bond angle of NCl3 is greater than NH3 as Pπ − dπ back-bonding is present in NCl3.b)BF3 is more acidic than BCl3 due to presence of Pπ − dπ back bonding in BF3c)When the bond multiplicity is increased, bond angle is decreased.d)The bond angle of SiCl4 is greater than CCl4Correct answer is option 'A'. Can you explain this answer?
Question Description
Valence shell electron-pair repulsion theory predicts the shape of a molecule by considering the most stable configuration of the bond angles in the molecule. The main points of the theory are(i) Electron pairs in the valence shell of central atom of a molecule, whether bonding or lone pairs are regarded as occupying localised orbitals. These orbitals arrange themselves so as to minimize the mutual electronic repulsions.(ii) The magnitude of the different types of electronic repulsions follow the order given below:Lone pair-lone pair> lone pair-bonding pair > bonding pair-bonding pair. These repulsive forces alter the bond angles of the molecule or ion.(iii) The electron repulsion between two pairs of electrons will be minimum if they stay as far as possible. On this basis, several studied. have been doneWhich of the following statements is correct ?a)The bond angle of NCl3 is greater than NH3 as Pπ − dπ back-bonding is present in NCl3.b)BF3 is more acidic than BCl3 due to presence of Pπ − dπ back bonding in BF3c)When the bond multiplicity is increased, bond angle is decreased.d)The bond angle of SiCl4 is greater than CCl4Correct answer is option 'A'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Valence shell electron-pair repulsion theory predicts the shape of a molecule by considering the most stable configuration of the bond angles in the molecule. The main points of the theory are(i) Electron pairs in the valence shell of central atom of a molecule, whether bonding or lone pairs are regarded as occupying localised orbitals. These orbitals arrange themselves so as to minimize the mutual electronic repulsions.(ii) The magnitude of the different types of electronic repulsions follow the order given below:Lone pair-lone pair> lone pair-bonding pair > bonding pair-bonding pair. These repulsive forces alter the bond angles of the molecule or ion.(iii) The electron repulsion between two pairs of electrons will be minimum if they stay as far as possible. On this basis, several studied. have been doneWhich of the following statements is correct ?a)The bond angle of NCl3 is greater than NH3 as Pπ − dπ back-bonding is present in NCl3.b)BF3 is more acidic than BCl3 due to presence of Pπ − dπ back bonding in BF3c)When the bond multiplicity is increased, bond angle is decreased.d)The bond angle of SiCl4 is greater than CCl4Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Valence shell electron-pair repulsion theory predicts the shape of a molecule by considering the most stable configuration of the bond angles in the molecule. The main points of the theory are(i) Electron pairs in the valence shell of central atom of a molecule, whether bonding or lone pairs are regarded as occupying localised orbitals. These orbitals arrange themselves so as to minimize the mutual electronic repulsions.(ii) The magnitude of the different types of electronic repulsions follow the order given below:Lone pair-lone pair> lone pair-bonding pair > bonding pair-bonding pair. These repulsive forces alter the bond angles of the molecule or ion.(iii) The electron repulsion between two pairs of electrons will be minimum if they stay as far as possible. On this basis, several studied. have been doneWhich of the following statements is correct ?a)The bond angle of NCl3 is greater than NH3 as Pπ − dπ back-bonding is present in NCl3.b)BF3 is more acidic than BCl3 due to presence of Pπ − dπ back bonding in BF3c)When the bond multiplicity is increased, bond angle is decreased.d)The bond angle of SiCl4 is greater than CCl4Correct answer is option 'A'. Can you explain this answer?.
Valence shell electron-pair repulsion theory predicts the shape of a molecule by considering the most stable configuration of the bond angles in the molecule. The main points of the theory are(i) Electron pairs in the valence shell of central atom of a molecule, whether bonding or lone pairs are regarded as occupying localised orbitals. These orbitals arrange themselves so as to minimize the mutual electronic repulsions.(ii) The magnitude of the different types of electronic repulsions follow the order given below:Lone pair-lone pair> lone pair-bonding pair > bonding pair-bonding pair. These repulsive forces alter the bond angles of the molecule or ion.(iii) The electron repulsion between two pairs of electrons will be minimum if they stay as far as possible. On this basis, several studied. have been doneWhich of the following statements is correct ?a)The bond angle of NCl3 is greater than NH3 as Pπ − dπ back-bonding is present in NCl3.b)BF3 is more acidic than BCl3 due to presence of Pπ − dπ back bonding in BF3c)When the bond multiplicity is increased, bond angle is decreased.d)The bond angle of SiCl4 is greater than CCl4Correct answer is option 'A'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Valence shell electron-pair repulsion theory predicts the shape of a molecule by considering the most stable configuration of the bond angles in the molecule. The main points of the theory are(i) Electron pairs in the valence shell of central atom of a molecule, whether bonding or lone pairs are regarded as occupying localised orbitals. These orbitals arrange themselves so as to minimize the mutual electronic repulsions.(ii) The magnitude of the different types of electronic repulsions follow the order given below:Lone pair-lone pair> lone pair-bonding pair > bonding pair-bonding pair. These repulsive forces alter the bond angles of the molecule or ion.(iii) The electron repulsion between two pairs of electrons will be minimum if they stay as far as possible. On this basis, several studied. have been doneWhich of the following statements is correct ?a)The bond angle of NCl3 is greater than NH3 as Pπ − dπ back-bonding is present in NCl3.b)BF3 is more acidic than BCl3 due to presence of Pπ − dπ back bonding in BF3c)When the bond multiplicity is increased, bond angle is decreased.d)The bond angle of SiCl4 is greater than CCl4Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Valence shell electron-pair repulsion theory predicts the shape of a molecule by considering the most stable configuration of the bond angles in the molecule. The main points of the theory are(i) Electron pairs in the valence shell of central atom of a molecule, whether bonding or lone pairs are regarded as occupying localised orbitals. These orbitals arrange themselves so as to minimize the mutual electronic repulsions.(ii) The magnitude of the different types of electronic repulsions follow the order given below:Lone pair-lone pair> lone pair-bonding pair > bonding pair-bonding pair. These repulsive forces alter the bond angles of the molecule or ion.(iii) The electron repulsion between two pairs of electrons will be minimum if they stay as far as possible. On this basis, several studied. have been doneWhich of the following statements is correct ?a)The bond angle of NCl3 is greater than NH3 as Pπ − dπ back-bonding is present in NCl3.b)BF3 is more acidic than BCl3 due to presence of Pπ − dπ back bonding in BF3c)When the bond multiplicity is increased, bond angle is decreased.d)The bond angle of SiCl4 is greater than CCl4Correct answer is option 'A'. Can you explain this answer?.
Solutions for Valence shell electron-pair repulsion theory predicts the shape of a molecule by considering the most stable configuration of the bond angles in the molecule. The main points of the theory are(i) Electron pairs in the valence shell of central atom of a molecule, whether bonding or lone pairs are regarded as occupying localised orbitals. These orbitals arrange themselves so as to minimize the mutual electronic repulsions.(ii) The magnitude of the different types of electronic repulsions follow the order given below:Lone pair-lone pair> lone pair-bonding pair > bonding pair-bonding pair. These repulsive forces alter the bond angles of the molecule or ion.(iii) The electron repulsion between two pairs of electrons will be minimum if they stay as far as possible. On this basis, several studied. have been doneWhich of the following statements is correct ?a)The bond angle of NCl3 is greater than NH3 as Pπ − dπ back-bonding is present in NCl3.b)BF3 is more acidic than BCl3 due to presence of Pπ − dπ back bonding in BF3c)When the bond multiplicity is increased, bond angle is decreased.d)The bond angle of SiCl4 is greater than CCl4Correct answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Valence shell electron-pair repulsion theory predicts the shape of a molecule by considering the most stable configuration of the bond angles in the molecule. The main points of the theory are(i) Electron pairs in the valence shell of central atom of a molecule, whether bonding or lone pairs are regarded as occupying localised orbitals. These orbitals arrange themselves so as to minimize the mutual electronic repulsions.(ii) The magnitude of the different types of electronic repulsions follow the order given below:Lone pair-lone pair> lone pair-bonding pair > bonding pair-bonding pair. These repulsive forces alter the bond angles of the molecule or ion.(iii) The electron repulsion between two pairs of electrons will be minimum if they stay as far as possible. On this basis, several studied. have been doneWhich of the following statements is correct ?a)The bond angle of NCl3 is greater than NH3 as Pπ − dπ back-bonding is present in NCl3.b)BF3 is more acidic than BCl3 due to presence of Pπ − dπ back bonding in BF3c)When the bond multiplicity is increased, bond angle is decreased.d)The bond angle of SiCl4 is greater than CCl4Correct answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Valence shell electron-pair repulsion theory predicts the shape of a molecule by considering the most stable configuration of the bond angles in the molecule. The main points of the theory are(i) Electron pairs in the valence shell of central atom of a molecule, whether bonding or lone pairs are regarded as occupying localised orbitals. These orbitals arrange themselves so as to minimize the mutual electronic repulsions.(ii) The magnitude of the different types of electronic repulsions follow the order given below:Lone pair-lone pair> lone pair-bonding pair > bonding pair-bonding pair. These repulsive forces alter the bond angles of the molecule or ion.(iii) The electron repulsion between two pairs of electrons will be minimum if they stay as far as possible. On this basis, several studied. have been doneWhich of the following statements is correct ?a)The bond angle of NCl3 is greater than NH3 as Pπ − dπ back-bonding is present in NCl3.b)BF3 is more acidic than BCl3 due to presence of Pπ − dπ back bonding in BF3c)When the bond multiplicity is increased, bond angle is decreased.d)The bond angle of SiCl4 is greater than CCl4Correct answer is option 'A'. Can you explain this answer?, a detailed solution for Valence shell electron-pair repulsion theory predicts the shape of a molecule by considering the most stable configuration of the bond angles in the molecule. The main points of the theory are(i) Electron pairs in the valence shell of central atom of a molecule, whether bonding or lone pairs are regarded as occupying localised orbitals. These orbitals arrange themselves so as to minimize the mutual electronic repulsions.(ii) The magnitude of the different types of electronic repulsions follow the order given below:Lone pair-lone pair> lone pair-bonding pair > bonding pair-bonding pair. These repulsive forces alter the bond angles of the molecule or ion.(iii) The electron repulsion between two pairs of electrons will be minimum if they stay as far as possible. On this basis, several studied. have been doneWhich of the following statements is correct ?a)The bond angle of NCl3 is greater than NH3 as Pπ − dπ back-bonding is present in NCl3.b)BF3 is more acidic than BCl3 due to presence of Pπ − dπ back bonding in BF3c)When the bond multiplicity is increased, bond angle is decreased.d)The bond angle of SiCl4 is greater than CCl4Correct answer is option 'A'. Can you explain this answer? has been provided alongside types of Valence shell electron-pair repulsion theory predicts the shape of a molecule by considering the most stable configuration of the bond angles in the molecule. The main points of the theory are(i) Electron pairs in the valence shell of central atom of a molecule, whether bonding or lone pairs are regarded as occupying localised orbitals. These orbitals arrange themselves so as to minimize the mutual electronic repulsions.(ii) The magnitude of the different types of electronic repulsions follow the order given below:Lone pair-lone pair> lone pair-bonding pair > bonding pair-bonding pair. These repulsive forces alter the bond angles of the molecule or ion.(iii) The electron repulsion between two pairs of electrons will be minimum if they stay as far as possible. On this basis, several studied. have been doneWhich of the following statements is correct ?a)The bond angle of NCl3 is greater than NH3 as Pπ − dπ back-bonding is present in NCl3.b)BF3 is more acidic than BCl3 due to presence of Pπ − dπ back bonding in BF3c)When the bond multiplicity is increased, bond angle is decreased.d)The bond angle of SiCl4 is greater than CCl4Correct answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Valence shell electron-pair repulsion theory predicts the shape of a molecule by considering the most stable configuration of the bond angles in the molecule. The main points of the theory are(i) Electron pairs in the valence shell of central atom of a molecule, whether bonding or lone pairs are regarded as occupying localised orbitals. These orbitals arrange themselves so as to minimize the mutual electronic repulsions.(ii) The magnitude of the different types of electronic repulsions follow the order given below:Lone pair-lone pair> lone pair-bonding pair > bonding pair-bonding pair. These repulsive forces alter the bond angles of the molecule or ion.(iii) The electron repulsion between two pairs of electrons will be minimum if they stay as far as possible. On this basis, several studied. have been doneWhich of the following statements is correct ?a)The bond angle of NCl3 is greater than NH3 as Pπ − dπ back-bonding is present in NCl3.b)BF3 is more acidic than BCl3 due to presence of Pπ − dπ back bonding in BF3c)When the bond multiplicity is increased, bond angle is decreased.d)The bond angle of SiCl4 is greater than CCl4Correct answer is option 'A'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























