JEE Exam > JEE Questions > Valence shell electron pair repulsion theory ...
Start Learning for Free
Valence shell electron pair repulsion theory (VSEPR) provides a method for predicting the shape of molecules, based on the electron pair electrostatic repulsion. According to VSEPR model, molecules adopt geometries in which their valence electron pairs position themselves as far from each other as possible. The VSEPR model considers double and triple bonds to have slightly greater repulsive effects than single bonds because of the repulsive effect of π electrons. However, the lone pair creates the maximum repulsive effect.
Q. Which species has the maximum number of lone pairs of electrons on the central atom?
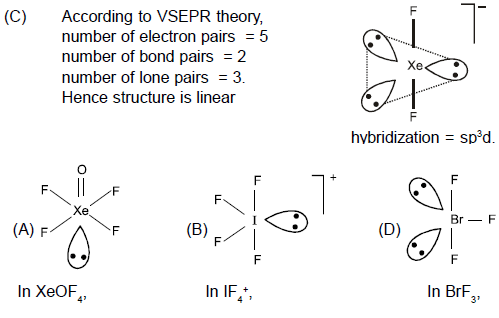
- a)XeOF4
- b)IF4+
- c)XeF2
- d)BrF3
Correct answer is option 'C'. Can you explain this answer?
Most Upvoted Answer
Valence shell electron pair repulsion theory (VSEPR) provides a method...
Understanding the VSEPR Theory
The Valence Shell Electron Pair Repulsion (VSEPR) theory helps predict the spatial arrangement of electron pairs around a central atom, focusing on minimizing repulsion between electron pairs.
Analyzing the Given Species
To determine which species has the maximum number of lone pairs on the central atom, we will analyze each option:
Conclusion
Among the given options, XeF2 has the highest number of lone pairs on the central atom, with 3 lone pairs. Thus, the correct answer is option 'C', XeF2.
The Valence Shell Electron Pair Repulsion (VSEPR) theory helps predict the spatial arrangement of electron pairs around a central atom, focusing on minimizing repulsion between electron pairs.
Analyzing the Given Species
To determine which species has the maximum number of lone pairs on the central atom, we will analyze each option:
- XeOF4:
- Central atom: Xenon (Xe)
- Valence electrons: 8 (Xe) + 6 (O) + 4 (4 F) = 18
- Bonding pairs: 4 (Xe-F) + 1 (Xe-O) = 5
- Lone pairs: 18 - 10 = 8 (1 lone pair on Xe) - IF4+:
- Central atom: Iodine (I)
- Valence electrons: 7 (I) + 4 (4 F) - 1 (charge) = 10
- Bonding pairs: 4 (I-F)
- Lone pairs: 10 - 8 = 2 (2 lone pairs on I) - XeF2:
- Central atom: Xenon (Xe)
- Valence electrons: 8 (Xe) + 2 (2 F) = 10
- Bonding pairs: 2 (Xe-F)
- Lone pairs: 10 - 4 = 6 (3 lone pairs on Xe) - BrF3:
- Central atom: Bromine (Br)
- Valence electrons: 7 (Br) + 3 (3 F) = 10
- Bonding pairs: 3 (Br-F)
- Lone pairs: 10 - 6 = 4 (2 lone pairs on Br)
Conclusion
Among the given options, XeF2 has the highest number of lone pairs on the central atom, with 3 lone pairs. Thus, the correct answer is option 'C', XeF2.
Free Test
FREE
| Start Free Test |
Community Answer
Valence shell electron pair repulsion theory (VSEPR) provides a method...

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Question Description
Valence shell electron pair repulsion theory (VSEPR) provides a method for predicting the shape of molecules, based on the electron pair electrostatic repulsion. According to VSEPR model, molecules adopt geometries in which their valence electron pairs position themselves as far from each other as possible. The VSEPR model considers double and triple bonds to have slightly greater repulsive effects than single bonds because of the repulsive effect of π electrons. However, the lone pair creates the maximum repulsive effect.Q.Which species has the maximum number of lone pairs of electrons on the central atom?a)XeOF4b)IF4+c)XeF2d)BrF3Correct answer is option 'C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Valence shell electron pair repulsion theory (VSEPR) provides a method for predicting the shape of molecules, based on the electron pair electrostatic repulsion. According to VSEPR model, molecules adopt geometries in which their valence electron pairs position themselves as far from each other as possible. The VSEPR model considers double and triple bonds to have slightly greater repulsive effects than single bonds because of the repulsive effect of π electrons. However, the lone pair creates the maximum repulsive effect.Q.Which species has the maximum number of lone pairs of electrons on the central atom?a)XeOF4b)IF4+c)XeF2d)BrF3Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Valence shell electron pair repulsion theory (VSEPR) provides a method for predicting the shape of molecules, based on the electron pair electrostatic repulsion. According to VSEPR model, molecules adopt geometries in which their valence electron pairs position themselves as far from each other as possible. The VSEPR model considers double and triple bonds to have slightly greater repulsive effects than single bonds because of the repulsive effect of π electrons. However, the lone pair creates the maximum repulsive effect.Q.Which species has the maximum number of lone pairs of electrons on the central atom?a)XeOF4b)IF4+c)XeF2d)BrF3Correct answer is option 'C'. Can you explain this answer?.
Valence shell electron pair repulsion theory (VSEPR) provides a method for predicting the shape of molecules, based on the electron pair electrostatic repulsion. According to VSEPR model, molecules adopt geometries in which their valence electron pairs position themselves as far from each other as possible. The VSEPR model considers double and triple bonds to have slightly greater repulsive effects than single bonds because of the repulsive effect of π electrons. However, the lone pair creates the maximum repulsive effect.Q.Which species has the maximum number of lone pairs of electrons on the central atom?a)XeOF4b)IF4+c)XeF2d)BrF3Correct answer is option 'C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Valence shell electron pair repulsion theory (VSEPR) provides a method for predicting the shape of molecules, based on the electron pair electrostatic repulsion. According to VSEPR model, molecules adopt geometries in which their valence electron pairs position themselves as far from each other as possible. The VSEPR model considers double and triple bonds to have slightly greater repulsive effects than single bonds because of the repulsive effect of π electrons. However, the lone pair creates the maximum repulsive effect.Q.Which species has the maximum number of lone pairs of electrons on the central atom?a)XeOF4b)IF4+c)XeF2d)BrF3Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Valence shell electron pair repulsion theory (VSEPR) provides a method for predicting the shape of molecules, based on the electron pair electrostatic repulsion. According to VSEPR model, molecules adopt geometries in which their valence electron pairs position themselves as far from each other as possible. The VSEPR model considers double and triple bonds to have slightly greater repulsive effects than single bonds because of the repulsive effect of π electrons. However, the lone pair creates the maximum repulsive effect.Q.Which species has the maximum number of lone pairs of electrons on the central atom?a)XeOF4b)IF4+c)XeF2d)BrF3Correct answer is option 'C'. Can you explain this answer?.
Solutions for Valence shell electron pair repulsion theory (VSEPR) provides a method for predicting the shape of molecules, based on the electron pair electrostatic repulsion. According to VSEPR model, molecules adopt geometries in which their valence electron pairs position themselves as far from each other as possible. The VSEPR model considers double and triple bonds to have slightly greater repulsive effects than single bonds because of the repulsive effect of π electrons. However, the lone pair creates the maximum repulsive effect.Q.Which species has the maximum number of lone pairs of electrons on the central atom?a)XeOF4b)IF4+c)XeF2d)BrF3Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Valence shell electron pair repulsion theory (VSEPR) provides a method for predicting the shape of molecules, based on the electron pair electrostatic repulsion. According to VSEPR model, molecules adopt geometries in which their valence electron pairs position themselves as far from each other as possible. The VSEPR model considers double and triple bonds to have slightly greater repulsive effects than single bonds because of the repulsive effect of π electrons. However, the lone pair creates the maximum repulsive effect.Q.Which species has the maximum number of lone pairs of electrons on the central atom?a)XeOF4b)IF4+c)XeF2d)BrF3Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Valence shell electron pair repulsion theory (VSEPR) provides a method for predicting the shape of molecules, based on the electron pair electrostatic repulsion. According to VSEPR model, molecules adopt geometries in which their valence electron pairs position themselves as far from each other as possible. The VSEPR model considers double and triple bonds to have slightly greater repulsive effects than single bonds because of the repulsive effect of π electrons. However, the lone pair creates the maximum repulsive effect.Q.Which species has the maximum number of lone pairs of electrons on the central atom?a)XeOF4b)IF4+c)XeF2d)BrF3Correct answer is option 'C'. Can you explain this answer?, a detailed solution for Valence shell electron pair repulsion theory (VSEPR) provides a method for predicting the shape of molecules, based on the electron pair electrostatic repulsion. According to VSEPR model, molecules adopt geometries in which their valence electron pairs position themselves as far from each other as possible. The VSEPR model considers double and triple bonds to have slightly greater repulsive effects than single bonds because of the repulsive effect of π electrons. However, the lone pair creates the maximum repulsive effect.Q.Which species has the maximum number of lone pairs of electrons on the central atom?a)XeOF4b)IF4+c)XeF2d)BrF3Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of Valence shell electron pair repulsion theory (VSEPR) provides a method for predicting the shape of molecules, based on the electron pair electrostatic repulsion. According to VSEPR model, molecules adopt geometries in which their valence electron pairs position themselves as far from each other as possible. The VSEPR model considers double and triple bonds to have slightly greater repulsive effects than single bonds because of the repulsive effect of π electrons. However, the lone pair creates the maximum repulsive effect.Q.Which species has the maximum number of lone pairs of electrons on the central atom?a)XeOF4b)IF4+c)XeF2d)BrF3Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Valence shell electron pair repulsion theory (VSEPR) provides a method for predicting the shape of molecules, based on the electron pair electrostatic repulsion. According to VSEPR model, molecules adopt geometries in which their valence electron pairs position themselves as far from each other as possible. The VSEPR model considers double and triple bonds to have slightly greater repulsive effects than single bonds because of the repulsive effect of π electrons. However, the lone pair creates the maximum repulsive effect.Q.Which species has the maximum number of lone pairs of electrons on the central atom?a)XeOF4b)IF4+c)XeF2d)BrF3Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



























