JEE Exam > JEE Questions > Ni + H2SO4 (hot and concentrated) → X(g).The...
Start Learning for Free
Ni + H2SO4 (hot and concentrated) → X(g).The liberated gas __________.
- a)develops blue spots on the filter paper moistened with potassium iodate and starch solution
- b)turns acidified K2Cr2O7 solution green
- c)produces black precipitates with lead acetate solution
- d)reacts with Cl2 water to produce an acid that produces white fumes with ammonia
Correct answer is option 'A,B,D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
Ni + H2SO4 (hot and concentrated) → X(g).The liberated gas __________...
The given reaction is: Ni + H2SO4 (hot and concentrated) → X(g)
In this reaction, a gas is liberated. Let's analyze the options one by one to determine the properties of the liberated gas.
a) The gas develops blue spots on filter paper moistened with potassium iodate and starch solution.
This observation suggests the presence of iodine. Iodine is commonly detected using a starch test, where it reacts with potassium iodate (KIO3) in the presence of starch to form a deep blue complex. Since the liberated gas forms blue spots on the filter paper, it indicates the presence of iodine. Therefore, option 'A' is correct.
b) The liberated gas turns acidified K2Cr2O7 solution green.
Potassium dichromate (K2Cr2O7) is a powerful oxidizing agent. When it reacts with reducing agents, such as iodine, it gets reduced, resulting in a color change from orange to green. Since the liberated gas turns acidified K2Cr2O7 solution green, it confirms the presence of iodine. Therefore, option 'B' is correct.
c) The liberated gas produces black precipitates with lead acetate solution.
Lead acetate solution is commonly used to detect the presence of hydrogen sulfide (H2S). When H2S reacts with lead acetate, it forms a black precipitate of lead sulfide (PbS). However, the given reaction involves H2SO4 (sulfuric acid) and not H2S. Therefore, option 'C' is not correct.
d) The liberated gas reacts with Cl2 water to produce an acid that produces white fumes with ammonia.
The reaction between the liberated gas and Cl2 water indicates the presence of a strong oxidizing agent. This eliminates the possibility of hydrogen gas (H2) being the liberated gas since it is a reducing agent. The only gas that fits this description is chlorine (Cl2). When chlorine reacts with water, it forms hydrochloric acid (HCl) and releases chlorine gas (Cl2). This chlorine gas reacts with ammonia (NH3) to produce white fumes of ammonium chloride (NH4Cl). Therefore, option 'D' is correct.
To summarize:
- The liberated gas develops blue spots on the filter paper moistened with potassium iodate and starch solution, indicating the presence of iodine (option 'A').
- The liberated gas turns acidified K2Cr2O7 solution green, confirming the presence of iodine (option 'B').
- The liberated gas does not produce black precipitates with lead acetate solution, eliminating option 'C'.
- The liberated gas reacts with Cl2 water to produce an acid that produces white fumes with ammonia, indicating the presence of chlorine (option 'D').
In this reaction, a gas is liberated. Let's analyze the options one by one to determine the properties of the liberated gas.
a) The gas develops blue spots on filter paper moistened with potassium iodate and starch solution.
This observation suggests the presence of iodine. Iodine is commonly detected using a starch test, where it reacts with potassium iodate (KIO3) in the presence of starch to form a deep blue complex. Since the liberated gas forms blue spots on the filter paper, it indicates the presence of iodine. Therefore, option 'A' is correct.
b) The liberated gas turns acidified K2Cr2O7 solution green.
Potassium dichromate (K2Cr2O7) is a powerful oxidizing agent. When it reacts with reducing agents, such as iodine, it gets reduced, resulting in a color change from orange to green. Since the liberated gas turns acidified K2Cr2O7 solution green, it confirms the presence of iodine. Therefore, option 'B' is correct.
c) The liberated gas produces black precipitates with lead acetate solution.
Lead acetate solution is commonly used to detect the presence of hydrogen sulfide (H2S). When H2S reacts with lead acetate, it forms a black precipitate of lead sulfide (PbS). However, the given reaction involves H2SO4 (sulfuric acid) and not H2S. Therefore, option 'C' is not correct.
d) The liberated gas reacts with Cl2 water to produce an acid that produces white fumes with ammonia.
The reaction between the liberated gas and Cl2 water indicates the presence of a strong oxidizing agent. This eliminates the possibility of hydrogen gas (H2) being the liberated gas since it is a reducing agent. The only gas that fits this description is chlorine (Cl2). When chlorine reacts with water, it forms hydrochloric acid (HCl) and releases chlorine gas (Cl2). This chlorine gas reacts with ammonia (NH3) to produce white fumes of ammonium chloride (NH4Cl). Therefore, option 'D' is correct.
To summarize:
- The liberated gas develops blue spots on the filter paper moistened with potassium iodate and starch solution, indicating the presence of iodine (option 'A').
- The liberated gas turns acidified K2Cr2O7 solution green, confirming the presence of iodine (option 'B').
- The liberated gas does not produce black precipitates with lead acetate solution, eliminating option 'C'.
- The liberated gas reacts with Cl2 water to produce an acid that produces white fumes with ammonia, indicating the presence of chlorine (option 'D').
Free Test
FREE
| Start Free Test |
Community Answer
Ni + H2SO4 (hot and concentrated) → X(g).The liberated gas __________...
Sulphur dioxide is produced by the reaction of Ni and concentrated H2SO4. In acidic medium, potassium iodate is reduced to iodide by sodium sulphite. The reaction takes place through the following steps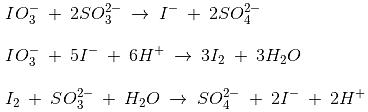
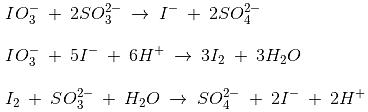
When sulphite ions are completely consumed, the liberated iodine would react with starch solution to give a blue colour.
(2) If the colourless gas evolved during the preliminary test is brought in contact with the filter paper moistened with acidified potassium dichromate solution, then the paper would turn green.
SO2 + K2Cr2O7 → Cr2(SO4)3 (green)
(3) Cl2 + H2O → HCl
HCl + NH3 → NH4Cl (white fumes)
Attention JEE Students!
To make sure you are not studying endlessly, EduRev has designed JEE study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in JEE.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Ni + H2SO4 (hot and concentrated) → X(g).The liberated gas __________.a)develops blue spots on the filter paper moistened with potassium iodate and starch solutionb)turns acidified K2Cr2O7 solution greenc)produces black precipitates with lead acetate solutiond)reacts with Cl2 water to produce an acid that produces white fumes with ammoniaCorrect answer is option 'A,B,D'. Can you explain this answer?
Question Description
Ni + H2SO4 (hot and concentrated) → X(g).The liberated gas __________.a)develops blue spots on the filter paper moistened with potassium iodate and starch solutionb)turns acidified K2Cr2O7 solution greenc)produces black precipitates with lead acetate solutiond)reacts with Cl2 water to produce an acid that produces white fumes with ammoniaCorrect answer is option 'A,B,D'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Ni + H2SO4 (hot and concentrated) → X(g).The liberated gas __________.a)develops blue spots on the filter paper moistened with potassium iodate and starch solutionb)turns acidified K2Cr2O7 solution greenc)produces black precipitates with lead acetate solutiond)reacts with Cl2 water to produce an acid that produces white fumes with ammoniaCorrect answer is option 'A,B,D'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Ni + H2SO4 (hot and concentrated) → X(g).The liberated gas __________.a)develops blue spots on the filter paper moistened with potassium iodate and starch solutionb)turns acidified K2Cr2O7 solution greenc)produces black precipitates with lead acetate solutiond)reacts with Cl2 water to produce an acid that produces white fumes with ammoniaCorrect answer is option 'A,B,D'. Can you explain this answer?.
Ni + H2SO4 (hot and concentrated) → X(g).The liberated gas __________.a)develops blue spots on the filter paper moistened with potassium iodate and starch solutionb)turns acidified K2Cr2O7 solution greenc)produces black precipitates with lead acetate solutiond)reacts with Cl2 water to produce an acid that produces white fumes with ammoniaCorrect answer is option 'A,B,D'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Ni + H2SO4 (hot and concentrated) → X(g).The liberated gas __________.a)develops blue spots on the filter paper moistened with potassium iodate and starch solutionb)turns acidified K2Cr2O7 solution greenc)produces black precipitates with lead acetate solutiond)reacts with Cl2 water to produce an acid that produces white fumes with ammoniaCorrect answer is option 'A,B,D'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Ni + H2SO4 (hot and concentrated) → X(g).The liberated gas __________.a)develops blue spots on the filter paper moistened with potassium iodate and starch solutionb)turns acidified K2Cr2O7 solution greenc)produces black precipitates with lead acetate solutiond)reacts with Cl2 water to produce an acid that produces white fumes with ammoniaCorrect answer is option 'A,B,D'. Can you explain this answer?.
Solutions for Ni + H2SO4 (hot and concentrated) → X(g).The liberated gas __________.a)develops blue spots on the filter paper moistened with potassium iodate and starch solutionb)turns acidified K2Cr2O7 solution greenc)produces black precipitates with lead acetate solutiond)reacts with Cl2 water to produce an acid that produces white fumes with ammoniaCorrect answer is option 'A,B,D'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Ni + H2SO4 (hot and concentrated) → X(g).The liberated gas __________.a)develops blue spots on the filter paper moistened with potassium iodate and starch solutionb)turns acidified K2Cr2O7 solution greenc)produces black precipitates with lead acetate solutiond)reacts with Cl2 water to produce an acid that produces white fumes with ammoniaCorrect answer is option 'A,B,D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Ni + H2SO4 (hot and concentrated) → X(g).The liberated gas __________.a)develops blue spots on the filter paper moistened with potassium iodate and starch solutionb)turns acidified K2Cr2O7 solution greenc)produces black precipitates with lead acetate solutiond)reacts with Cl2 water to produce an acid that produces white fumes with ammoniaCorrect answer is option 'A,B,D'. Can you explain this answer?, a detailed solution for Ni + H2SO4 (hot and concentrated) → X(g).The liberated gas __________.a)develops blue spots on the filter paper moistened with potassium iodate and starch solutionb)turns acidified K2Cr2O7 solution greenc)produces black precipitates with lead acetate solutiond)reacts with Cl2 water to produce an acid that produces white fumes with ammoniaCorrect answer is option 'A,B,D'. Can you explain this answer? has been provided alongside types of Ni + H2SO4 (hot and concentrated) → X(g).The liberated gas __________.a)develops blue spots on the filter paper moistened with potassium iodate and starch solutionb)turns acidified K2Cr2O7 solution greenc)produces black precipitates with lead acetate solutiond)reacts with Cl2 water to produce an acid that produces white fumes with ammoniaCorrect answer is option 'A,B,D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Ni + H2SO4 (hot and concentrated) → X(g).The liberated gas __________.a)develops blue spots on the filter paper moistened with potassium iodate and starch solutionb)turns acidified K2Cr2O7 solution greenc)produces black precipitates with lead acetate solutiond)reacts with Cl2 water to produce an acid that produces white fumes with ammoniaCorrect answer is option 'A,B,D'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























