JEE Exam > JEE Questions > The correct statement(s) related to colloids ...
Start Learning for Free
The correct statement(s) related to colloids is/are
- a)The process of precipitating colloidal sol by an electrolyte is called peptization.
- b)Colloidal solution freezes at higher temperature than the true solution at the same concentration.
- c)Surfactants form micelle above critical micelle concentration (CMC). CMC depends on temperature.
- d)Micelles are macromolecular colloids.
Correct answer is option 'B,C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
The correct statement(s) related to colloids is/area)The process of pr...
Colloids:
Colloids are the mixtures in which the size of the particles is intermediate between those of true solutions and suspensions. The particles of colloids are not visible to the naked eye but can be seen under an ultramicroscope.
Correct statement(s) related to colloids are:
B) Colloidal solution freezes at a higher temperature than the true solution at the same concentration. This is because the particles in the colloidal solution have a larger surface area than the particles in the true solution, which results in more energy being required to freeze the colloidal solution.
C) Surfactants form micelle above critical micelle concentration (CMC). CMC depends on temperature. The surfactant molecules in the solution arrange themselves in a spherical structure called a micelle when their concentration reaches a certain level known as the critical micelle concentration (CMC). The CMC depends on the temperature of the solution.
The other statements are incorrect:
A) The process of precipitating colloidal sol by an electrolyte is called peptization. This is incorrect as peptization is the process of converting a precipitate into a colloidal sol by adding a suitable electrolyte.
D) Micelles are macromolecular colloids. This is incorrect as micelles are not macromolecular colloids. They are aggregates of surfactant molecules that form a spherical structure in a solution.
In conclusion, the correct statements related to colloids are that colloidal solutions freeze at a higher temperature than true solutions at the same concentration and that surfactants form micelles above the critical micelle concentration, which depends on temperature.
Colloids are the mixtures in which the size of the particles is intermediate between those of true solutions and suspensions. The particles of colloids are not visible to the naked eye but can be seen under an ultramicroscope.
Correct statement(s) related to colloids are:
B) Colloidal solution freezes at a higher temperature than the true solution at the same concentration. This is because the particles in the colloidal solution have a larger surface area than the particles in the true solution, which results in more energy being required to freeze the colloidal solution.
C) Surfactants form micelle above critical micelle concentration (CMC). CMC depends on temperature. The surfactant molecules in the solution arrange themselves in a spherical structure called a micelle when their concentration reaches a certain level known as the critical micelle concentration (CMC). The CMC depends on the temperature of the solution.
The other statements are incorrect:
A) The process of precipitating colloidal sol by an electrolyte is called peptization. This is incorrect as peptization is the process of converting a precipitate into a colloidal sol by adding a suitable electrolyte.
D) Micelles are macromolecular colloids. This is incorrect as micelles are not macromolecular colloids. They are aggregates of surfactant molecules that form a spherical structure in a solution.
In conclusion, the correct statements related to colloids are that colloidal solutions freeze at a higher temperature than true solutions at the same concentration and that surfactants form micelles above the critical micelle concentration, which depends on temperature.
Free Test
FREE
| Start Free Test |
Community Answer
The correct statement(s) related to colloids is/area)The process of pr...
(1) Process of precipitating colloidal solution by using an electrolyte is called 'coagulation' and not peptization.
(2) Since molar mass of sol is much higher than true solutions, magnitude of any colligative properties is smaller than true solutions.
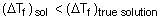
So, freezing point of sols > freezing point of true solution
So, option (2) is correct.
(3) Micelles are formed at greater than or equal to CMC and above KRAFT temperature.
So, option (3) is also correct.
(4) Micelles are associated colloids and not macromolecular colloids.
(2) Since molar mass of sol is much higher than true solutions, magnitude of any colligative properties is smaller than true solutions.
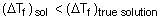
So, freezing point of sols > freezing point of true solution
So, option (2) is correct.
(3) Micelles are formed at greater than or equal to CMC and above KRAFT temperature.
So, option (3) is also correct.
(4) Micelles are associated colloids and not macromolecular colloids.
Attention JEE Students!
To make sure you are not studying endlessly, EduRev has designed JEE study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in JEE.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
The correct statement(s) related to colloids is/area)The process of precipitating colloidal sol by an electrolyte is called peptization.b)Colloidal solution freezes at higher temperature than the true solution at the same concentration.c)Surfactants form micelle above critical micelle concentration (CMC). CMC depends on temperature.d)Micelles are macromolecular colloids.Correct answer is option 'B,C'. Can you explain this answer?
Question Description
The correct statement(s) related to colloids is/area)The process of precipitating colloidal sol by an electrolyte is called peptization.b)Colloidal solution freezes at higher temperature than the true solution at the same concentration.c)Surfactants form micelle above critical micelle concentration (CMC). CMC depends on temperature.d)Micelles are macromolecular colloids.Correct answer is option 'B,C'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about The correct statement(s) related to colloids is/area)The process of precipitating colloidal sol by an electrolyte is called peptization.b)Colloidal solution freezes at higher temperature than the true solution at the same concentration.c)Surfactants form micelle above critical micelle concentration (CMC). CMC depends on temperature.d)Micelles are macromolecular colloids.Correct answer is option 'B,C'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The correct statement(s) related to colloids is/area)The process of precipitating colloidal sol by an electrolyte is called peptization.b)Colloidal solution freezes at higher temperature than the true solution at the same concentration.c)Surfactants form micelle above critical micelle concentration (CMC). CMC depends on temperature.d)Micelles are macromolecular colloids.Correct answer is option 'B,C'. Can you explain this answer?.
The correct statement(s) related to colloids is/area)The process of precipitating colloidal sol by an electrolyte is called peptization.b)Colloidal solution freezes at higher temperature than the true solution at the same concentration.c)Surfactants form micelle above critical micelle concentration (CMC). CMC depends on temperature.d)Micelles are macromolecular colloids.Correct answer is option 'B,C'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about The correct statement(s) related to colloids is/area)The process of precipitating colloidal sol by an electrolyte is called peptization.b)Colloidal solution freezes at higher temperature than the true solution at the same concentration.c)Surfactants form micelle above critical micelle concentration (CMC). CMC depends on temperature.d)Micelles are macromolecular colloids.Correct answer is option 'B,C'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The correct statement(s) related to colloids is/area)The process of precipitating colloidal sol by an electrolyte is called peptization.b)Colloidal solution freezes at higher temperature than the true solution at the same concentration.c)Surfactants form micelle above critical micelle concentration (CMC). CMC depends on temperature.d)Micelles are macromolecular colloids.Correct answer is option 'B,C'. Can you explain this answer?.
Solutions for The correct statement(s) related to colloids is/area)The process of precipitating colloidal sol by an electrolyte is called peptization.b)Colloidal solution freezes at higher temperature than the true solution at the same concentration.c)Surfactants form micelle above critical micelle concentration (CMC). CMC depends on temperature.d)Micelles are macromolecular colloids.Correct answer is option 'B,C'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of The correct statement(s) related to colloids is/area)The process of precipitating colloidal sol by an electrolyte is called peptization.b)Colloidal solution freezes at higher temperature than the true solution at the same concentration.c)Surfactants form micelle above critical micelle concentration (CMC). CMC depends on temperature.d)Micelles are macromolecular colloids.Correct answer is option 'B,C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The correct statement(s) related to colloids is/area)The process of precipitating colloidal sol by an electrolyte is called peptization.b)Colloidal solution freezes at higher temperature than the true solution at the same concentration.c)Surfactants form micelle above critical micelle concentration (CMC). CMC depends on temperature.d)Micelles are macromolecular colloids.Correct answer is option 'B,C'. Can you explain this answer?, a detailed solution for The correct statement(s) related to colloids is/area)The process of precipitating colloidal sol by an electrolyte is called peptization.b)Colloidal solution freezes at higher temperature than the true solution at the same concentration.c)Surfactants form micelle above critical micelle concentration (CMC). CMC depends on temperature.d)Micelles are macromolecular colloids.Correct answer is option 'B,C'. Can you explain this answer? has been provided alongside types of The correct statement(s) related to colloids is/area)The process of precipitating colloidal sol by an electrolyte is called peptization.b)Colloidal solution freezes at higher temperature than the true solution at the same concentration.c)Surfactants form micelle above critical micelle concentration (CMC). CMC depends on temperature.d)Micelles are macromolecular colloids.Correct answer is option 'B,C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The correct statement(s) related to colloids is/area)The process of precipitating colloidal sol by an electrolyte is called peptization.b)Colloidal solution freezes at higher temperature than the true solution at the same concentration.c)Surfactants form micelle above critical micelle concentration (CMC). CMC depends on temperature.d)Micelles are macromolecular colloids.Correct answer is option 'B,C'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























