JEE Exam > JEE Questions > A proton, a neutron, an electron and an &alph...
Start Learning for Free
A proton, a neutron, an electron and an α-particle have same energy. If λp, λn, λe and λα are the de-Broglie's wavelengths of proton, neutron, electron and α particle, respectively then choose the correct relation from the following:
- a)λp = λn > λe > λα
- b)λα < λn < λp < λe
- c)λe < λp = λn > λα
- d)λe = λp = λn = λα
Correct answer is option 'B'. Can you explain this answer?
Most Upvoted Answer
A proton, a neutron, an electron and an α-particle have same ene...
Alpha particle walk into a bar.
The proton orders a pint of beer and starts chatting with the bartender about the latest scientific discoveries. The neutron orders a glass of water and joins the conversation, adding some interesting facts about nuclear physics.
The electron, being a bit of a troublemaker, orders a shot of tequila and starts dancing on the bar counter, creating a vibrant light show. The bartender tries to calm the electron down, but it's too late. The electron's wild dance moves knock over some glasses and cause a small electrical surge.
Just as the bartender is about to kick the electron out, the alpha particle walks in. The alpha particle is composed of two protons and two neutrons tightly bound together. It takes one look at the chaos caused by the electron and immediately goes into action.
The alpha particle gently approaches the electron and starts pulling it away from the bar, using its strong nuclear force to keep the electron in check. The electron, realizing it's outmatched, reluctantly follows the alpha particle's lead.
The bartender breathes a sigh of relief as the alpha particle successfully escorts the electron out of the bar, restoring peace and order. The proton and neutron thank the alpha particle for its help and continue their scientific discussion with the bartender, now enjoying a calm and quiet evening at the bar.
The proton orders a pint of beer and starts chatting with the bartender about the latest scientific discoveries. The neutron orders a glass of water and joins the conversation, adding some interesting facts about nuclear physics.
The electron, being a bit of a troublemaker, orders a shot of tequila and starts dancing on the bar counter, creating a vibrant light show. The bartender tries to calm the electron down, but it's too late. The electron's wild dance moves knock over some glasses and cause a small electrical surge.
Just as the bartender is about to kick the electron out, the alpha particle walks in. The alpha particle is composed of two protons and two neutrons tightly bound together. It takes one look at the chaos caused by the electron and immediately goes into action.
The alpha particle gently approaches the electron and starts pulling it away from the bar, using its strong nuclear force to keep the electron in check. The electron, realizing it's outmatched, reluctantly follows the alpha particle's lead.
The bartender breathes a sigh of relief as the alpha particle successfully escorts the electron out of the bar, restoring peace and order. The proton and neutron thank the alpha particle for its help and continue their scientific discussion with the bartender, now enjoying a calm and quiet evening at the bar.
Free Test
FREE
| Start Free Test |
Community Answer
A proton, a neutron, an electron and an α-particle have same ene...
de Broglie wavelength λ = h/p
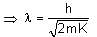
Where K: kinetic energy
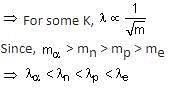
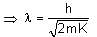
Where K: kinetic energy
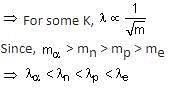

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
A proton, a neutron, an electron and an α-particle have same energy. If λp,λn,λeandλα are the de-Broglies wavelengths of proton, neutron, electron and αparticle, respectively then choose the correct relation from the following:a)λp =λn >λe >λαb)λα< λn< λp< λec)λe <λp =λn >λαd)λe =λp =λn =λαCorrect answer is option 'B'. Can you explain this answer?
Question Description
A proton, a neutron, an electron and an α-particle have same energy. If λp,λn,λeandλα are the de-Broglies wavelengths of proton, neutron, electron and αparticle, respectively then choose the correct relation from the following:a)λp =λn >λe >λαb)λα< λn< λp< λec)λe <λp =λn >λαd)λe =λp =λn =λαCorrect answer is option 'B'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about A proton, a neutron, an electron and an α-particle have same energy. If λp,λn,λeandλα are the de-Broglies wavelengths of proton, neutron, electron and αparticle, respectively then choose the correct relation from the following:a)λp =λn >λe >λαb)λα< λn< λp< λec)λe <λp =λn >λαd)λe =λp =λn =λαCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A proton, a neutron, an electron and an α-particle have same energy. If λp,λn,λeandλα are the de-Broglies wavelengths of proton, neutron, electron and αparticle, respectively then choose the correct relation from the following:a)λp =λn >λe >λαb)λα< λn< λp< λec)λe <λp =λn >λαd)λe =λp =λn =λαCorrect answer is option 'B'. Can you explain this answer?.
A proton, a neutron, an electron and an α-particle have same energy. If λp,λn,λeandλα are the de-Broglies wavelengths of proton, neutron, electron and αparticle, respectively then choose the correct relation from the following:a)λp =λn >λe >λαb)λα< λn< λp< λec)λe <λp =λn >λαd)λe =λp =λn =λαCorrect answer is option 'B'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about A proton, a neutron, an electron and an α-particle have same energy. If λp,λn,λeandλα are the de-Broglies wavelengths of proton, neutron, electron and αparticle, respectively then choose the correct relation from the following:a)λp =λn >λe >λαb)λα< λn< λp< λec)λe <λp =λn >λαd)λe =λp =λn =λαCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A proton, a neutron, an electron and an α-particle have same energy. If λp,λn,λeandλα are the de-Broglies wavelengths of proton, neutron, electron and αparticle, respectively then choose the correct relation from the following:a)λp =λn >λe >λαb)λα< λn< λp< λec)λe <λp =λn >λαd)λe =λp =λn =λαCorrect answer is option 'B'. Can you explain this answer?.
Solutions for A proton, a neutron, an electron and an α-particle have same energy. If λp,λn,λeandλα are the de-Broglies wavelengths of proton, neutron, electron and αparticle, respectively then choose the correct relation from the following:a)λp =λn >λe >λαb)λα< λn< λp< λec)λe <λp =λn >λαd)λe =λp =λn =λαCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of A proton, a neutron, an electron and an α-particle have same energy. If λp,λn,λeandλα are the de-Broglies wavelengths of proton, neutron, electron and αparticle, respectively then choose the correct relation from the following:a)λp =λn >λe >λαb)λα< λn< λp< λec)λe <λp =λn >λαd)λe =λp =λn =λαCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
A proton, a neutron, an electron and an α-particle have same energy. If λp,λn,λeandλα are the de-Broglies wavelengths of proton, neutron, electron and αparticle, respectively then choose the correct relation from the following:a)λp =λn >λe >λαb)λα< λn< λp< λec)λe <λp =λn >λαd)λe =λp =λn =λαCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for A proton, a neutron, an electron and an α-particle have same energy. If λp,λn,λeandλα are the de-Broglies wavelengths of proton, neutron, electron and αparticle, respectively then choose the correct relation from the following:a)λp =λn >λe >λαb)λα< λn< λp< λec)λe <λp =λn >λαd)λe =λp =λn =λαCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of A proton, a neutron, an electron and an α-particle have same energy. If λp,λn,λeandλα are the de-Broglies wavelengths of proton, neutron, electron and αparticle, respectively then choose the correct relation from the following:a)λp =λn >λe >λαb)λα< λn< λp< λec)λe <λp =λn >λαd)λe =λp =λn =λαCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice A proton, a neutron, an electron and an α-particle have same energy. If λp,λn,λeandλα are the de-Broglies wavelengths of proton, neutron, electron and αparticle, respectively then choose the correct relation from the following:a)λp =λn >λe >λαb)λα< λn< λp< λec)λe <λp =λn >λαd)λe =λp =λn =λαCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























