Civil Engineering (CE) Exam > Civil Engineering (CE) Questions > When chlorine is dissolved in water, it react...
Start Learning for Free
When chlorine is dissolved in water, it reacts to form hypochlorous acid and hypochlorite ions. At pH < 5, chlorine exists in water as
- a)Elemental or molecular chlorine
- b)Remains in the form of hypochlorous acid
- c)Remains in the form of hypochlorite ions
- d)Remains in the form of both hypochlorous acid and hypochlorite ions
Correct answer is option 'A'. Can you explain this answer?
Most Upvoted Answer
When chlorine is dissolved in water, it reacts to form hypochlorous ac...
Elemental or molecular chlorine
Chlorine exists in water as elemental or molecular chlorine at pH < 5.="" this="" means="" that="" chlorine="" remains="" in="" its="" original="" form="" rather="" than="" reacting="" to="" form="" hypochlorous="" acid="" and="" hypochlorite="" ions.="" />
- At low pH levels, the majority of the chlorine remains in its molecular form due to the lack of sufficient hydroxide ions to facilitate the formation of hypochlorous acid and hypochlorite ions.
- This is important to consider in water treatment processes where the pH of the water can impact the effectiveness of chlorine as a disinfectant.
- In order to ensure proper disinfection, adjustments may need to be made to the pH of the water to promote the formation of hypochlorous acid, which is a more potent disinfectant compared to molecular chlorine.
Overall, understanding the behavior of chlorine in water at different pH levels is crucial for effective water treatment and disinfection processes.
Free Test
FREE
| Start Free Test |
Community Answer
When chlorine is dissolved in water, it reacts to form hypochlorous ac...
Concept:
In the process of chlorination chlorine reacts with water to form Hypochlorous acid as given in the below equation.
In the process of chlorination chlorine reacts with water to form Hypochlorous acid as given in the below equation.
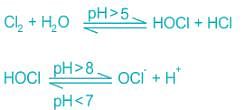
At pH < 5, chlorine does not react with water and remains in elemental or molecular chlorine form.
Other important points:
Effectivity in disinfection-
ClO2> HOCl> Chloroamines> Hypochlorite ion (OCl-)
Some of the dechlorinating agents are -
Effectivity in disinfection-
ClO2> HOCl> Chloroamines> Hypochlorite ion (OCl-)
Some of the dechlorinating agents are -
- Na2S2O3 (Sodium thiosulphate)
- Na2S2O5 (Sodium metabisulphate)
- Na2HSO3 (Sodium bisulphide)
- SO2 and Activated carbon

|
Explore Courses for Civil Engineering (CE) exam
|

|
When chlorine is dissolved in water, it reacts to form hypochlorous acid and hypochlorite ions. At pH < 5, chlorine exists in water asa)Elemental or molecular chlorineb)Remains in the form of hypochlorous acidc)Remains in the form of hypochlorite ionsd)Remains in the form of both hypochlorous acid and hypochlorite ionsCorrect answer is option 'A'. Can you explain this answer?
Question Description
When chlorine is dissolved in water, it reacts to form hypochlorous acid and hypochlorite ions. At pH < 5, chlorine exists in water asa)Elemental or molecular chlorineb)Remains in the form of hypochlorous acidc)Remains in the form of hypochlorite ionsd)Remains in the form of both hypochlorous acid and hypochlorite ionsCorrect answer is option 'A'. Can you explain this answer? for Civil Engineering (CE) 2025 is part of Civil Engineering (CE) preparation. The Question and answers have been prepared according to the Civil Engineering (CE) exam syllabus. Information about When chlorine is dissolved in water, it reacts to form hypochlorous acid and hypochlorite ions. At pH < 5, chlorine exists in water asa)Elemental or molecular chlorineb)Remains in the form of hypochlorous acidc)Remains in the form of hypochlorite ionsd)Remains in the form of both hypochlorous acid and hypochlorite ionsCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Civil Engineering (CE) 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When chlorine is dissolved in water, it reacts to form hypochlorous acid and hypochlorite ions. At pH < 5, chlorine exists in water asa)Elemental or molecular chlorineb)Remains in the form of hypochlorous acidc)Remains in the form of hypochlorite ionsd)Remains in the form of both hypochlorous acid and hypochlorite ionsCorrect answer is option 'A'. Can you explain this answer?.
When chlorine is dissolved in water, it reacts to form hypochlorous acid and hypochlorite ions. At pH < 5, chlorine exists in water asa)Elemental or molecular chlorineb)Remains in the form of hypochlorous acidc)Remains in the form of hypochlorite ionsd)Remains in the form of both hypochlorous acid and hypochlorite ionsCorrect answer is option 'A'. Can you explain this answer? for Civil Engineering (CE) 2025 is part of Civil Engineering (CE) preparation. The Question and answers have been prepared according to the Civil Engineering (CE) exam syllabus. Information about When chlorine is dissolved in water, it reacts to form hypochlorous acid and hypochlorite ions. At pH < 5, chlorine exists in water asa)Elemental or molecular chlorineb)Remains in the form of hypochlorous acidc)Remains in the form of hypochlorite ionsd)Remains in the form of both hypochlorous acid and hypochlorite ionsCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for Civil Engineering (CE) 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When chlorine is dissolved in water, it reacts to form hypochlorous acid and hypochlorite ions. At pH < 5, chlorine exists in water asa)Elemental or molecular chlorineb)Remains in the form of hypochlorous acidc)Remains in the form of hypochlorite ionsd)Remains in the form of both hypochlorous acid and hypochlorite ionsCorrect answer is option 'A'. Can you explain this answer?.
Solutions for When chlorine is dissolved in water, it reacts to form hypochlorous acid and hypochlorite ions. At pH < 5, chlorine exists in water asa)Elemental or molecular chlorineb)Remains in the form of hypochlorous acidc)Remains in the form of hypochlorite ionsd)Remains in the form of both hypochlorous acid and hypochlorite ionsCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Civil Engineering (CE).
Download more important topics, notes, lectures and mock test series for Civil Engineering (CE) Exam by signing up for free.
Here you can find the meaning of When chlorine is dissolved in water, it reacts to form hypochlorous acid and hypochlorite ions. At pH < 5, chlorine exists in water asa)Elemental or molecular chlorineb)Remains in the form of hypochlorous acidc)Remains in the form of hypochlorite ionsd)Remains in the form of both hypochlorous acid and hypochlorite ionsCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
When chlorine is dissolved in water, it reacts to form hypochlorous acid and hypochlorite ions. At pH < 5, chlorine exists in water asa)Elemental or molecular chlorineb)Remains in the form of hypochlorous acidc)Remains in the form of hypochlorite ionsd)Remains in the form of both hypochlorous acid and hypochlorite ionsCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for When chlorine is dissolved in water, it reacts to form hypochlorous acid and hypochlorite ions. At pH < 5, chlorine exists in water asa)Elemental or molecular chlorineb)Remains in the form of hypochlorous acidc)Remains in the form of hypochlorite ionsd)Remains in the form of both hypochlorous acid and hypochlorite ionsCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of When chlorine is dissolved in water, it reacts to form hypochlorous acid and hypochlorite ions. At pH < 5, chlorine exists in water asa)Elemental or molecular chlorineb)Remains in the form of hypochlorous acidc)Remains in the form of hypochlorite ionsd)Remains in the form of both hypochlorous acid and hypochlorite ionsCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice When chlorine is dissolved in water, it reacts to form hypochlorous acid and hypochlorite ions. At pH < 5, chlorine exists in water asa)Elemental or molecular chlorineb)Remains in the form of hypochlorous acidc)Remains in the form of hypochlorite ionsd)Remains in the form of both hypochlorous acid and hypochlorite ionsCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice Civil Engineering (CE) tests.

|
Explore Courses for Civil Engineering (CE) exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























