JEE Exam > JEE Questions > At 25°C, 50 g of iron reacts with HCI to ...
Start Learning for Free
At 25°C, 50 g of iron reacts with HCI to form FeCl2. The evolved hydrogen gas expands against a constant pressure of 1 bar. The work done by the gas during this expansion is ________ J.
(Round off to the nearest integer) [Given: R = 8.314 J mol-1 K-1. Assume, hydrogen is an ideal gas]
[Atomic mass of Fe is 55.85 u]
(Round off to the nearest integer) [Given: R = 8.314 J mol-1 K-1. Assume, hydrogen is an ideal gas]
[Atomic mass of Fe is 55.85 u]
Correct answer is '2218'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
At 25°C, 50 g of iron reacts with HCI to form FeCl2. The evolved h...
T = 298 K, R = 8.314 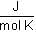
→ Chemicl reaction is:
Fe + 2HCI → FeCl2 + H2(g)
50g P = 1 bar
= 50/55.85 mol
→ Work done for 1 mol gas
= -Pext × ΔV
= Δ ng RT
= -1 × 8.314 × 298 J
→ Work done for 50/55.85 mol gas
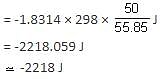
When work done is negative, expansion of the gas takes place.
So, the correct answer is 2218.
→ Chemicl reaction is:
Fe + 2HCI → FeCl2 + H2(g)
50g P = 1 bar
= 50/55.85 mol
→ Work done for 1 mol gas
= -Pext × ΔV
= Δ ng RT
= -1 × 8.314 × 298 J
→ Work done for 50/55.85 mol gas
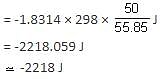
When work done is negative, expansion of the gas takes place.
So, the correct answer is 2218.
Free Test
FREE
| Start Free Test |
Community Answer
At 25°C, 50 g of iron reacts with HCI to form FeCl2. The evolved h...
At 25 years old, many individuals are transitioning from their early 20s into adulthood. This can be a time of self-discovery, personal growth, and significant life changes. Here are some common experiences and considerations for someone at 25:
1. Career and Education: Many people have completed their education and are starting their careers or pursuing further education. This may involve job hunting, internships, or graduate school.
2. Financial Independence: At this age, individuals may strive for financial independence, managing their own expenses, and saving for the future. They might also be paying off student loans or other debts.
3. Relationships: Some individuals may be in committed relationships or considering settling down. This can involve navigating the complexities of long-term partnerships, marriage, or starting a family.
4. Housing: Many people at 25 are transitioning from living with roommates or in shared spaces to having their own apartments or houses. This can involve finding a suitable place to live and managing household responsibilities.
5. Personal Development: This is a period of self-discovery and personal growth. People may be exploring their interests, hobbies, and passions while also developing their identity and values.
6. Health and Well-being: Taking care of physical and mental well-being becomes more important. This includes maintaining a healthy lifestyle, seeking medical check-ups, and managing stress.
7. Social Life: Friendships are an important part of this stage, and individuals may be building new connections while also maintaining existing friendships. People often engage in social activities, hobbies, and community involvement.
8. Future Planning: At 25, individuals may start considering long-term goals and making plans for their future. This can involve setting career objectives, creating a financial roadmap, and envisioning personal aspirations.
It is important to note that everyone's experiences are unique, and individuals may have different priorities, challenges, and accomplishments at this age.
1. Career and Education: Many people have completed their education and are starting their careers or pursuing further education. This may involve job hunting, internships, or graduate school.
2. Financial Independence: At this age, individuals may strive for financial independence, managing their own expenses, and saving for the future. They might also be paying off student loans or other debts.
3. Relationships: Some individuals may be in committed relationships or considering settling down. This can involve navigating the complexities of long-term partnerships, marriage, or starting a family.
4. Housing: Many people at 25 are transitioning from living with roommates or in shared spaces to having their own apartments or houses. This can involve finding a suitable place to live and managing household responsibilities.
5. Personal Development: This is a period of self-discovery and personal growth. People may be exploring their interests, hobbies, and passions while also developing their identity and values.
6. Health and Well-being: Taking care of physical and mental well-being becomes more important. This includes maintaining a healthy lifestyle, seeking medical check-ups, and managing stress.
7. Social Life: Friendships are an important part of this stage, and individuals may be building new connections while also maintaining existing friendships. People often engage in social activities, hobbies, and community involvement.
8. Future Planning: At 25, individuals may start considering long-term goals and making plans for their future. This can involve setting career objectives, creating a financial roadmap, and envisioning personal aspirations.
It is important to note that everyone's experiences are unique, and individuals may have different priorities, challenges, and accomplishments at this age.
Attention JEE Students!
To make sure you are not studying endlessly, EduRev has designed JEE study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in JEE.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
At 25°C, 50 g of iron reacts with HCI to form FeCl2. The evolved hydrogen gas expands against a constant pressure of 1 bar. The work done by the gas during this expansion is ________ J.(Round off to the nearest integer) [Given: R = 8.314 J mol-1 K-1. Assume, hydrogen is an ideal gas][Atomic mass of Fe is 55.85 u]Correct answer is '2218'. Can you explain this answer?
Question Description
At 25°C, 50 g of iron reacts with HCI to form FeCl2. The evolved hydrogen gas expands against a constant pressure of 1 bar. The work done by the gas during this expansion is ________ J.(Round off to the nearest integer) [Given: R = 8.314 J mol-1 K-1. Assume, hydrogen is an ideal gas][Atomic mass of Fe is 55.85 u]Correct answer is '2218'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about At 25°C, 50 g of iron reacts with HCI to form FeCl2. The evolved hydrogen gas expands against a constant pressure of 1 bar. The work done by the gas during this expansion is ________ J.(Round off to the nearest integer) [Given: R = 8.314 J mol-1 K-1. Assume, hydrogen is an ideal gas][Atomic mass of Fe is 55.85 u]Correct answer is '2218'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for At 25°C, 50 g of iron reacts with HCI to form FeCl2. The evolved hydrogen gas expands against a constant pressure of 1 bar. The work done by the gas during this expansion is ________ J.(Round off to the nearest integer) [Given: R = 8.314 J mol-1 K-1. Assume, hydrogen is an ideal gas][Atomic mass of Fe is 55.85 u]Correct answer is '2218'. Can you explain this answer?.
At 25°C, 50 g of iron reacts with HCI to form FeCl2. The evolved hydrogen gas expands against a constant pressure of 1 bar. The work done by the gas during this expansion is ________ J.(Round off to the nearest integer) [Given: R = 8.314 J mol-1 K-1. Assume, hydrogen is an ideal gas][Atomic mass of Fe is 55.85 u]Correct answer is '2218'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about At 25°C, 50 g of iron reacts with HCI to form FeCl2. The evolved hydrogen gas expands against a constant pressure of 1 bar. The work done by the gas during this expansion is ________ J.(Round off to the nearest integer) [Given: R = 8.314 J mol-1 K-1. Assume, hydrogen is an ideal gas][Atomic mass of Fe is 55.85 u]Correct answer is '2218'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for At 25°C, 50 g of iron reacts with HCI to form FeCl2. The evolved hydrogen gas expands against a constant pressure of 1 bar. The work done by the gas during this expansion is ________ J.(Round off to the nearest integer) [Given: R = 8.314 J mol-1 K-1. Assume, hydrogen is an ideal gas][Atomic mass of Fe is 55.85 u]Correct answer is '2218'. Can you explain this answer?.
Solutions for At 25°C, 50 g of iron reacts with HCI to form FeCl2. The evolved hydrogen gas expands against a constant pressure of 1 bar. The work done by the gas during this expansion is ________ J.(Round off to the nearest integer) [Given: R = 8.314 J mol-1 K-1. Assume, hydrogen is an ideal gas][Atomic mass of Fe is 55.85 u]Correct answer is '2218'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of At 25°C, 50 g of iron reacts with HCI to form FeCl2. The evolved hydrogen gas expands against a constant pressure of 1 bar. The work done by the gas during this expansion is ________ J.(Round off to the nearest integer) [Given: R = 8.314 J mol-1 K-1. Assume, hydrogen is an ideal gas][Atomic mass of Fe is 55.85 u]Correct answer is '2218'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
At 25°C, 50 g of iron reacts with HCI to form FeCl2. The evolved hydrogen gas expands against a constant pressure of 1 bar. The work done by the gas during this expansion is ________ J.(Round off to the nearest integer) [Given: R = 8.314 J mol-1 K-1. Assume, hydrogen is an ideal gas][Atomic mass of Fe is 55.85 u]Correct answer is '2218'. Can you explain this answer?, a detailed solution for At 25°C, 50 g of iron reacts with HCI to form FeCl2. The evolved hydrogen gas expands against a constant pressure of 1 bar. The work done by the gas during this expansion is ________ J.(Round off to the nearest integer) [Given: R = 8.314 J mol-1 K-1. Assume, hydrogen is an ideal gas][Atomic mass of Fe is 55.85 u]Correct answer is '2218'. Can you explain this answer? has been provided alongside types of At 25°C, 50 g of iron reacts with HCI to form FeCl2. The evolved hydrogen gas expands against a constant pressure of 1 bar. The work done by the gas during this expansion is ________ J.(Round off to the nearest integer) [Given: R = 8.314 J mol-1 K-1. Assume, hydrogen is an ideal gas][Atomic mass of Fe is 55.85 u]Correct answer is '2218'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice At 25°C, 50 g of iron reacts with HCI to form FeCl2. The evolved hydrogen gas expands against a constant pressure of 1 bar. The work done by the gas during this expansion is ________ J.(Round off to the nearest integer) [Given: R = 8.314 J mol-1 K-1. Assume, hydrogen is an ideal gas][Atomic mass of Fe is 55.85 u]Correct answer is '2218'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























