JEE Exam > JEE Questions > Given below are two statements:Statement I: H...
Start Learning for Free
Given below are two statements:
Statement I: H2O2 can act as both oxidising and reducing agent in basic medium.
Statement II: In the hydrogen economy, the energy is transmitted in the form of dihydrogen.
In the light of the above statements, choose the correct answer from the options given below:
Statement I: H2O2 can act as both oxidising and reducing agent in basic medium.
Statement II: In the hydrogen economy, the energy is transmitted in the form of dihydrogen.
In the light of the above statements, choose the correct answer from the options given below:
- a)Both statement I and statement II are false.
- b)Both statement I and statement II are true.
- c)Statement I is true, but statement II is false.
- d)Statement I is false, but statement II is true.
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Most Upvoted Answer
Given below are two statements:Statement I: H2O2 can act as both oxidi...
Statement I: H2O2 can act as both oxidising and reducing agent in basic medium.
H2O2 is commonly known as hydrogen peroxide. It is a powerful oxidizing and reducing agent, capable of undergoing both oxidation and reduction reactions. However, the nature of its action depends on the medium in which it is present.
In an acidic medium, H2O2 acts as a strong oxidizing agent. It readily donates oxygen atoms, which results in the oxidation of other substances. For example, in the reaction between H2O2 and Fe2+, H2O2 acts as an oxidizing agent and oxidizes Fe2+ to Fe3+.
2H2O2 + 2Fe2+ + 2H+ → 2Fe3+ + 4H2O
On the other hand, in a basic medium, H2O2 can act as a reducing agent. It readily accepts oxygen atoms, which results in the reduction of other substances. For example, in the reaction between H2O2 and MnO4-, H2O2 acts as a reducing agent and reduces MnO4- to Mn2+.
5H2O2 + 2MnO4- + 6OH- → 2Mn2+ + 5O2 + 8H2O
Therefore, statement I is true.
Statement II: In the hydrogen economy, the energy is transmitted in the form of dihydrogen.
The hydrogen economy refers to a hypothetical future energy system where hydrogen is used as a primary energy carrier. In this system, energy is stored and transmitted in the form of dihydrogen (H2) gas.
H2 is a clean and efficient fuel that can be produced from various renewable sources such as water and biomass. It can be used in fuel cells to generate electricity with only water as a byproduct. The idea behind the hydrogen economy is to replace fossil fuels with hydrogen as a sustainable and environmentally friendly energy source.
Therefore, statement II is also true.
Conclusion:
Both statement I and statement II are true. H2O2 can act as both an oxidizing and reducing agent in a basic medium, and in the hydrogen economy, energy is transmitted in the form of dihydrogen.
H2O2 is commonly known as hydrogen peroxide. It is a powerful oxidizing and reducing agent, capable of undergoing both oxidation and reduction reactions. However, the nature of its action depends on the medium in which it is present.
In an acidic medium, H2O2 acts as a strong oxidizing agent. It readily donates oxygen atoms, which results in the oxidation of other substances. For example, in the reaction between H2O2 and Fe2+, H2O2 acts as an oxidizing agent and oxidizes Fe2+ to Fe3+.
2H2O2 + 2Fe2+ + 2H+ → 2Fe3+ + 4H2O
On the other hand, in a basic medium, H2O2 can act as a reducing agent. It readily accepts oxygen atoms, which results in the reduction of other substances. For example, in the reaction between H2O2 and MnO4-, H2O2 acts as a reducing agent and reduces MnO4- to Mn2+.
5H2O2 + 2MnO4- + 6OH- → 2Mn2+ + 5O2 + 8H2O
Therefore, statement I is true.
Statement II: In the hydrogen economy, the energy is transmitted in the form of dihydrogen.
The hydrogen economy refers to a hypothetical future energy system where hydrogen is used as a primary energy carrier. In this system, energy is stored and transmitted in the form of dihydrogen (H2) gas.
H2 is a clean and efficient fuel that can be produced from various renewable sources such as water and biomass. It can be used in fuel cells to generate electricity with only water as a byproduct. The idea behind the hydrogen economy is to replace fossil fuels with hydrogen as a sustainable and environmentally friendly energy source.
Therefore, statement II is also true.
Conclusion:
Both statement I and statement II are true. H2O2 can act as both an oxidizing and reducing agent in a basic medium, and in the hydrogen economy, energy is transmitted in the form of dihydrogen.
Free Test
FREE
| Start Free Test |
Community Answer
Given below are two statements:Statement I: H2O2 can act as both oxidi...
(a) H2O2 can act as both oxidising and reducing agent in basic medium.
(i) 2Fe2+ + H2O2 → 2Fe3+ + 2OH-
In this reaction, H2O2 acts as an oxidising agent.
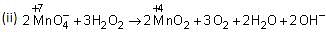
In this reaction, H2O2 acts as a reducing agent.
(b) The basic principle of hydrogen economy is the transportation and storage of energy in the form of liquids or gaseous dihydrogen.
Advantage of hydrogen economy is that energy is transmitted in the form of dihydrogen and not as electric power.
(i) 2Fe2+ + H2O2 → 2Fe3+ + 2OH-
In this reaction, H2O2 acts as an oxidising agent.
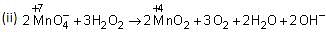
In this reaction, H2O2 acts as a reducing agent.
(b) The basic principle of hydrogen economy is the transportation and storage of energy in the form of liquids or gaseous dihydrogen.
Advantage of hydrogen economy is that energy is transmitted in the form of dihydrogen and not as electric power.
Attention JEE Students!
To make sure you are not studying endlessly, EduRev has designed JEE study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in JEE.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Given below are two statements:Statement I: H2O2 can act as both oxidising and reducing agent in basic medium.Statement II: In the hydrogen economy, the energy is transmitted in the form of dihydrogen.In the light of the above statements, choose the correct answer from the options given below:a)Both statement I and statement II are false.b)Both statement I and statement II are true.c)Statement I is true, but statement II is false.d)Statement I is false, but statement II is true.Correct answer is option 'B'. Can you explain this answer?
Question Description
Given below are two statements:Statement I: H2O2 can act as both oxidising and reducing agent in basic medium.Statement II: In the hydrogen economy, the energy is transmitted in the form of dihydrogen.In the light of the above statements, choose the correct answer from the options given below:a)Both statement I and statement II are false.b)Both statement I and statement II are true.c)Statement I is true, but statement II is false.d)Statement I is false, but statement II is true.Correct answer is option 'B'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Given below are two statements:Statement I: H2O2 can act as both oxidising and reducing agent in basic medium.Statement II: In the hydrogen economy, the energy is transmitted in the form of dihydrogen.In the light of the above statements, choose the correct answer from the options given below:a)Both statement I and statement II are false.b)Both statement I and statement II are true.c)Statement I is true, but statement II is false.d)Statement I is false, but statement II is true.Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Given below are two statements:Statement I: H2O2 can act as both oxidising and reducing agent in basic medium.Statement II: In the hydrogen economy, the energy is transmitted in the form of dihydrogen.In the light of the above statements, choose the correct answer from the options given below:a)Both statement I and statement II are false.b)Both statement I and statement II are true.c)Statement I is true, but statement II is false.d)Statement I is false, but statement II is true.Correct answer is option 'B'. Can you explain this answer?.
Given below are two statements:Statement I: H2O2 can act as both oxidising and reducing agent in basic medium.Statement II: In the hydrogen economy, the energy is transmitted in the form of dihydrogen.In the light of the above statements, choose the correct answer from the options given below:a)Both statement I and statement II are false.b)Both statement I and statement II are true.c)Statement I is true, but statement II is false.d)Statement I is false, but statement II is true.Correct answer is option 'B'. Can you explain this answer? for JEE 2024 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Given below are two statements:Statement I: H2O2 can act as both oxidising and reducing agent in basic medium.Statement II: In the hydrogen economy, the energy is transmitted in the form of dihydrogen.In the light of the above statements, choose the correct answer from the options given below:a)Both statement I and statement II are false.b)Both statement I and statement II are true.c)Statement I is true, but statement II is false.d)Statement I is false, but statement II is true.Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Given below are two statements:Statement I: H2O2 can act as both oxidising and reducing agent in basic medium.Statement II: In the hydrogen economy, the energy is transmitted in the form of dihydrogen.In the light of the above statements, choose the correct answer from the options given below:a)Both statement I and statement II are false.b)Both statement I and statement II are true.c)Statement I is true, but statement II is false.d)Statement I is false, but statement II is true.Correct answer is option 'B'. Can you explain this answer?.
Solutions for Given below are two statements:Statement I: H2O2 can act as both oxidising and reducing agent in basic medium.Statement II: In the hydrogen economy, the energy is transmitted in the form of dihydrogen.In the light of the above statements, choose the correct answer from the options given below:a)Both statement I and statement II are false.b)Both statement I and statement II are true.c)Statement I is true, but statement II is false.d)Statement I is false, but statement II is true.Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Given below are two statements:Statement I: H2O2 can act as both oxidising and reducing agent in basic medium.Statement II: In the hydrogen economy, the energy is transmitted in the form of dihydrogen.In the light of the above statements, choose the correct answer from the options given below:a)Both statement I and statement II are false.b)Both statement I and statement II are true.c)Statement I is true, but statement II is false.d)Statement I is false, but statement II is true.Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Given below are two statements:Statement I: H2O2 can act as both oxidising and reducing agent in basic medium.Statement II: In the hydrogen economy, the energy is transmitted in the form of dihydrogen.In the light of the above statements, choose the correct answer from the options given below:a)Both statement I and statement II are false.b)Both statement I and statement II are true.c)Statement I is true, but statement II is false.d)Statement I is false, but statement II is true.Correct answer is option 'B'. Can you explain this answer?, a detailed solution for Given below are two statements:Statement I: H2O2 can act as both oxidising and reducing agent in basic medium.Statement II: In the hydrogen economy, the energy is transmitted in the form of dihydrogen.In the light of the above statements, choose the correct answer from the options given below:a)Both statement I and statement II are false.b)Both statement I and statement II are true.c)Statement I is true, but statement II is false.d)Statement I is false, but statement II is true.Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of Given below are two statements:Statement I: H2O2 can act as both oxidising and reducing agent in basic medium.Statement II: In the hydrogen economy, the energy is transmitted in the form of dihydrogen.In the light of the above statements, choose the correct answer from the options given below:a)Both statement I and statement II are false.b)Both statement I and statement II are true.c)Statement I is true, but statement II is false.d)Statement I is false, but statement II is true.Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Given below are two statements:Statement I: H2O2 can act as both oxidising and reducing agent in basic medium.Statement II: In the hydrogen economy, the energy is transmitted in the form of dihydrogen.In the light of the above statements, choose the correct answer from the options given below:a)Both statement I and statement II are false.b)Both statement I and statement II are true.c)Statement I is true, but statement II is false.d)Statement I is false, but statement II is true.Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























