JEE Exam > JEE Questions > The redox reaction among the following is:a)R...
Start Learning for Free
The redox reaction among the following is:
- a)Reaction of [Co(H2O)6]Cl3 with AgNO3
- b)Formation of ozone from atmospheric oxygen in the presence of sunlight
- c)Combination of dinitrogen with dioxygen at 2000 K
- d)Reaction of H2SO4 with NaOH
Correct answer is option 'C'. Can you explain this answer?
Most Upvoted Answer
The redox reaction among the following is:a)Reaction of [Co(H2O)6]Cl3 ...
The redox reaction among the given options is the combination of dinitrogen (N2) with dioxygen (O2) at 2000 K.
Explanation:
Redox reactions involve the transfer of electrons between species. In these reactions, one species is oxidized (loses electrons) while another species is reduced (gains electrons). Let's analyze each option to identify the redox reaction:
a) Reaction of [Co(H2O)6]Cl3 with AgNO3:
In this reaction, the cobalt complex [Co(H2O)6]3+ is displaced by the silver ion, resulting in the formation of [Ag(H2O)6]3+ and Cl- ions. There is no change in the oxidation state of any element, so this is not a redox reaction.
b) Formation of ozone from atmospheric oxygen in the presence of sunlight:
The formation of ozone (O3) from oxygen (O2) in the presence of sunlight is a photochemical reaction. It involves the splitting of O2 molecules into individual oxygen atoms, which then combine with other O2 molecules to form ozone. This reaction does not involve any change in the oxidation state of oxygen, so it is not a redox reaction.
c) Combination of dinitrogen with dioxygen at 2000 K:
This is the redox reaction among the given options. At high temperatures (2000 K), nitrogen gas (N2) can react with oxygen gas (O2) to form nitrogen dioxide (NO2). In this reaction, nitrogen undergoes oxidation from an oxidation state of 0 in N2 to +4 in NO2, while oxygen undergoes reduction from an oxidation state of 0 in O2 to -2 in NO2.
d) Reaction of H2SO4 with NaOH:
When sulfuric acid (H2SO4) reacts with sodium hydroxide (NaOH), a neutralization reaction occurs. The hydrogen ions (H+) from sulfuric acid combine with the hydroxide ions (OH-) from sodium hydroxide to form water (H2O), and the remaining sodium ion (Na+) and sulfate ion (SO42-) combine to form sodium sulfate (Na2SO4). There is no change in the oxidation state of any element, so this is not a redox reaction.
In summary, the redox reaction among the given options is the combination of dinitrogen with dioxygen at 2000 K.
Explanation:
Redox reactions involve the transfer of electrons between species. In these reactions, one species is oxidized (loses electrons) while another species is reduced (gains electrons). Let's analyze each option to identify the redox reaction:
a) Reaction of [Co(H2O)6]Cl3 with AgNO3:
In this reaction, the cobalt complex [Co(H2O)6]3+ is displaced by the silver ion, resulting in the formation of [Ag(H2O)6]3+ and Cl- ions. There is no change in the oxidation state of any element, so this is not a redox reaction.
b) Formation of ozone from atmospheric oxygen in the presence of sunlight:
The formation of ozone (O3) from oxygen (O2) in the presence of sunlight is a photochemical reaction. It involves the splitting of O2 molecules into individual oxygen atoms, which then combine with other O2 molecules to form ozone. This reaction does not involve any change in the oxidation state of oxygen, so it is not a redox reaction.
c) Combination of dinitrogen with dioxygen at 2000 K:
This is the redox reaction among the given options. At high temperatures (2000 K), nitrogen gas (N2) can react with oxygen gas (O2) to form nitrogen dioxide (NO2). In this reaction, nitrogen undergoes oxidation from an oxidation state of 0 in N2 to +4 in NO2, while oxygen undergoes reduction from an oxidation state of 0 in O2 to -2 in NO2.
d) Reaction of H2SO4 with NaOH:
When sulfuric acid (H2SO4) reacts with sodium hydroxide (NaOH), a neutralization reaction occurs. The hydrogen ions (H+) from sulfuric acid combine with the hydroxide ions (OH-) from sodium hydroxide to form water (H2O), and the remaining sodium ion (Na+) and sulfate ion (SO42-) combine to form sodium sulfate (Na2SO4). There is no change in the oxidation state of any element, so this is not a redox reaction.
In summary, the redox reaction among the given options is the combination of dinitrogen with dioxygen at 2000 K.
Free Test
FREE
| Start Free Test |
Community Answer
The redox reaction among the following is:a)Reaction of [Co(H2O)6]Cl3 ...
The redox reaction is:
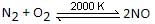
Nitrogen is oxidised, while oxygen is reduced. Reaction of [CO(H2O)6]Cl3 with AgNO3 is not a redox reaction. It is a precipitation reaction.
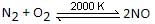
Nitrogen is oxidised, while oxygen is reduced. Reaction of [CO(H2O)6]Cl3 with AgNO3 is not a redox reaction. It is a precipitation reaction.

|
Explore Courses for JEE exam
|

|
Question Description
The redox reaction among the following is:a)Reaction of [Co(H2O)6]Cl3 with AgNO3b)Formation of ozone from atmospheric oxygen in the presence of sunlightc)Combination of dinitrogen with dioxygen at 2000 Kd)Reaction of H2SO4 with NaOHCorrect answer is option 'C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about The redox reaction among the following is:a)Reaction of [Co(H2O)6]Cl3 with AgNO3b)Formation of ozone from atmospheric oxygen in the presence of sunlightc)Combination of dinitrogen with dioxygen at 2000 Kd)Reaction of H2SO4 with NaOHCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The redox reaction among the following is:a)Reaction of [Co(H2O)6]Cl3 with AgNO3b)Formation of ozone from atmospheric oxygen in the presence of sunlightc)Combination of dinitrogen with dioxygen at 2000 Kd)Reaction of H2SO4 with NaOHCorrect answer is option 'C'. Can you explain this answer?.
The redox reaction among the following is:a)Reaction of [Co(H2O)6]Cl3 with AgNO3b)Formation of ozone from atmospheric oxygen in the presence of sunlightc)Combination of dinitrogen with dioxygen at 2000 Kd)Reaction of H2SO4 with NaOHCorrect answer is option 'C'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about The redox reaction among the following is:a)Reaction of [Co(H2O)6]Cl3 with AgNO3b)Formation of ozone from atmospheric oxygen in the presence of sunlightc)Combination of dinitrogen with dioxygen at 2000 Kd)Reaction of H2SO4 with NaOHCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The redox reaction among the following is:a)Reaction of [Co(H2O)6]Cl3 with AgNO3b)Formation of ozone from atmospheric oxygen in the presence of sunlightc)Combination of dinitrogen with dioxygen at 2000 Kd)Reaction of H2SO4 with NaOHCorrect answer is option 'C'. Can you explain this answer?.
Solutions for The redox reaction among the following is:a)Reaction of [Co(H2O)6]Cl3 with AgNO3b)Formation of ozone from atmospheric oxygen in the presence of sunlightc)Combination of dinitrogen with dioxygen at 2000 Kd)Reaction of H2SO4 with NaOHCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of The redox reaction among the following is:a)Reaction of [Co(H2O)6]Cl3 with AgNO3b)Formation of ozone from atmospheric oxygen in the presence of sunlightc)Combination of dinitrogen with dioxygen at 2000 Kd)Reaction of H2SO4 with NaOHCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The redox reaction among the following is:a)Reaction of [Co(H2O)6]Cl3 with AgNO3b)Formation of ozone from atmospheric oxygen in the presence of sunlightc)Combination of dinitrogen with dioxygen at 2000 Kd)Reaction of H2SO4 with NaOHCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for The redox reaction among the following is:a)Reaction of [Co(H2O)6]Cl3 with AgNO3b)Formation of ozone from atmospheric oxygen in the presence of sunlightc)Combination of dinitrogen with dioxygen at 2000 Kd)Reaction of H2SO4 with NaOHCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of The redox reaction among the following is:a)Reaction of [Co(H2O)6]Cl3 with AgNO3b)Formation of ozone from atmospheric oxygen in the presence of sunlightc)Combination of dinitrogen with dioxygen at 2000 Kd)Reaction of H2SO4 with NaOHCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The redox reaction among the following is:a)Reaction of [Co(H2O)6]Cl3 with AgNO3b)Formation of ozone from atmospheric oxygen in the presence of sunlightc)Combination of dinitrogen with dioxygen at 2000 Kd)Reaction of H2SO4 with NaOHCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























