UPPSC (UP) Exam > UPPSC (UP) Questions > The base used as an antacid isa)Calcium hydr...
Start Learning for Free
The base used as an antacid is
- a)Calcium hydroxide
- b)Barium hydroxide
- c)Magnesium hydroxide
- d)Silver hydroxide
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
The base used as an antacid isa)Calcium hydroxideb)Barium hydroxidec)...
An antacid is a substance which neutralizes stomach acidity. Antacids are bases used to neutralize the acid that causes heartburn. Despite the many commercial brand, almost all antacids act on excess stomach acid by neutralizing it with weak bases. The most common of these bases are hydroxides, carbonates, or bicarbonates. The following table contains a list of the active ingredients found in several common commercial antacids, and the reactions by which these antacids neutralize the HCl in stomach acid. The following are the main compound of antacids.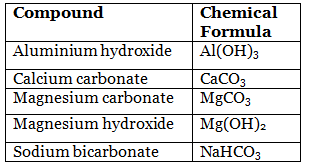
View all questions of this test

Most Upvoted Answer
The base used as an antacid isa)Calcium hydroxideb)Barium hydroxidec)...
The base used as an antacid is Magnesium hydroxide.
Antacids are medications that help to neutralize stomach acid, providing relief from symptoms such as heartburn, indigestion, and acid reflux. They work by increasing the pH level in the stomach, reducing the acidity and providing a soothing effect.
Magnesium hydroxide, also known as milk of magnesia, is a common antacid that is used to treat various stomach-related issues. Let's explore why it is the base of choice for this purpose:
1. Neutralization of acid:
- Stomach acid is primarily composed of hydrochloric acid (HCl).
- Magnesium hydroxide is a strong base that reacts with the acid in the stomach, forming water and a salt.
- The chemical equation for this reaction is: Mg(OH)2 + 2HCl → MgCl2 + 2H2O.
- The formation of water helps to neutralize the excess acid, reducing its corrosive effects on the stomach lining and providing relief from symptoms.
2. Magnesium content:
- Magnesium is an essential mineral that plays a crucial role in various bodily functions.
- Magnesium hydroxide contains a significant amount of magnesium, which can help to supplement the body's magnesium levels.
- Adequate magnesium levels are important for maintaining healthy nerve and muscle function, as well as supporting bone health.
3. Safety profile:
- Magnesium hydroxide is generally considered safe for short-term use as an antacid.
- It has a low risk of side effects, although some individuals may experience diarrhea or an upset stomach.
- However, it is important to use antacids as directed and not exceed the recommended dosage, as excessive use can lead to electrolyte imbalances and other complications.
4. Availability and affordability:
- Magnesium hydroxide is readily available over-the-counter in various forms, such as tablets, suspensions, and chewable tablets.
- It is also relatively affordable compared to other antacid options, making it accessible to a wide range of individuals.
In conclusion, Magnesium hydroxide is the base used as an antacid due to its ability to neutralize stomach acid, its magnesium content, its safety profile, and its availability and affordability. However, it is always recommended to consult a healthcare professional before starting any new medication or if symptoms persist.
Antacids are medications that help to neutralize stomach acid, providing relief from symptoms such as heartburn, indigestion, and acid reflux. They work by increasing the pH level in the stomach, reducing the acidity and providing a soothing effect.
Magnesium hydroxide, also known as milk of magnesia, is a common antacid that is used to treat various stomach-related issues. Let's explore why it is the base of choice for this purpose:
1. Neutralization of acid:
- Stomach acid is primarily composed of hydrochloric acid (HCl).
- Magnesium hydroxide is a strong base that reacts with the acid in the stomach, forming water and a salt.
- The chemical equation for this reaction is: Mg(OH)2 + 2HCl → MgCl2 + 2H2O.
- The formation of water helps to neutralize the excess acid, reducing its corrosive effects on the stomach lining and providing relief from symptoms.
2. Magnesium content:
- Magnesium is an essential mineral that plays a crucial role in various bodily functions.
- Magnesium hydroxide contains a significant amount of magnesium, which can help to supplement the body's magnesium levels.
- Adequate magnesium levels are important for maintaining healthy nerve and muscle function, as well as supporting bone health.
3. Safety profile:
- Magnesium hydroxide is generally considered safe for short-term use as an antacid.
- It has a low risk of side effects, although some individuals may experience diarrhea or an upset stomach.
- However, it is important to use antacids as directed and not exceed the recommended dosage, as excessive use can lead to electrolyte imbalances and other complications.
4. Availability and affordability:
- Magnesium hydroxide is readily available over-the-counter in various forms, such as tablets, suspensions, and chewable tablets.
- It is also relatively affordable compared to other antacid options, making it accessible to a wide range of individuals.
In conclusion, Magnesium hydroxide is the base used as an antacid due to its ability to neutralize stomach acid, its magnesium content, its safety profile, and its availability and affordability. However, it is always recommended to consult a healthcare professional before starting any new medication or if symptoms persist.
Free Test
FREE
| Start Free Test |
Community Answer
The base used as an antacid isa)Calcium hydroxideb)Barium hydroxidec)...
An antacid is a substance which neutralizes stomach acidity. Antacids are bases used to neutralize the acid that causes heartburn. Despite the many commercial brand, almost all antacids act on excess stomach acid by neutralizing it with weak bases. The most common of these bases are hydroxides, carbonates, or bicarbonates. The following table contains a list of the active ingredients found in several common commercial antacids, and the reactions by which these antacids neutralize the HCl in stomach acid. The following are the main compound of antacids.


|
Explore Courses for UPPSC (UP) exam
|

|
The base used as an antacid isa)Calcium hydroxideb)Barium hydroxidec)Magnesium hydroxided)Silver hydroxideCorrect answer is option 'C'. Can you explain this answer?
Question Description
The base used as an antacid isa)Calcium hydroxideb)Barium hydroxidec)Magnesium hydroxided)Silver hydroxideCorrect answer is option 'C'. Can you explain this answer? for UPPSC (UP) 2025 is part of UPPSC (UP) preparation. The Question and answers have been prepared according to the UPPSC (UP) exam syllabus. Information about The base used as an antacid isa)Calcium hydroxideb)Barium hydroxidec)Magnesium hydroxided)Silver hydroxideCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for UPPSC (UP) 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The base used as an antacid isa)Calcium hydroxideb)Barium hydroxidec)Magnesium hydroxided)Silver hydroxideCorrect answer is option 'C'. Can you explain this answer?.
The base used as an antacid isa)Calcium hydroxideb)Barium hydroxidec)Magnesium hydroxided)Silver hydroxideCorrect answer is option 'C'. Can you explain this answer? for UPPSC (UP) 2025 is part of UPPSC (UP) preparation. The Question and answers have been prepared according to the UPPSC (UP) exam syllabus. Information about The base used as an antacid isa)Calcium hydroxideb)Barium hydroxidec)Magnesium hydroxided)Silver hydroxideCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for UPPSC (UP) 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The base used as an antacid isa)Calcium hydroxideb)Barium hydroxidec)Magnesium hydroxided)Silver hydroxideCorrect answer is option 'C'. Can you explain this answer?.
Solutions for The base used as an antacid isa)Calcium hydroxideb)Barium hydroxidec)Magnesium hydroxided)Silver hydroxideCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for UPPSC (UP).
Download more important topics, notes, lectures and mock test series for UPPSC (UP) Exam by signing up for free.
Here you can find the meaning of The base used as an antacid isa)Calcium hydroxideb)Barium hydroxidec)Magnesium hydroxided)Silver hydroxideCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The base used as an antacid isa)Calcium hydroxideb)Barium hydroxidec)Magnesium hydroxided)Silver hydroxideCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for The base used as an antacid isa)Calcium hydroxideb)Barium hydroxidec)Magnesium hydroxided)Silver hydroxideCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of The base used as an antacid isa)Calcium hydroxideb)Barium hydroxidec)Magnesium hydroxided)Silver hydroxideCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The base used as an antacid isa)Calcium hydroxideb)Barium hydroxidec)Magnesium hydroxided)Silver hydroxideCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice UPPSC (UP) tests.

|
Explore Courses for UPPSC (UP) exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























