Class 11 Exam > Class 11 Questions > What is the difference between polar and the ...
Start Learning for Free
What is the difference between polar and the non polar molecular bond
Verified Answer
What is the difference between polar and the non polar molecular bond
 This question is part of UPSC exam. View all Class 11 courses
This question is part of UPSC exam. View all Class 11 courses
Most Upvoted Answer
What is the difference between polar and the non polar molecular bond
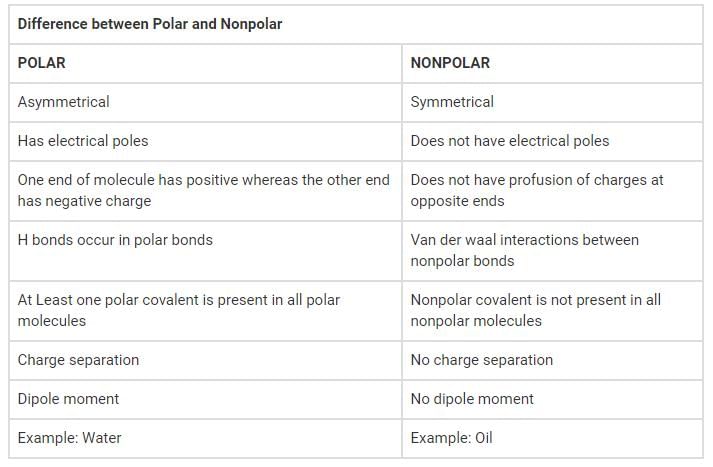
The Difference Between Polar and Nonpolar Molecular Bonds
Introduction:
Molecular bonds play a fundamental role in the formation and stability of compounds. These bonds can be classified as either polar or nonpolar based on the electronegativity difference between the atoms involved. Understanding the difference between polar and nonpolar bonds is crucial in comprehending the behavior and properties of various substances.
Polar Bonds:
A polar bond occurs when there is an unequal sharing of electrons between two atoms due to differences in electronegativity. Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond. In a polar bond, one atom has a higher electronegativity than the other, resulting in an uneven distribution of electron density.
Key Points:
- The more electronegative atom attracts the shared electrons closer to its nucleus, creating a partial negative charge.
- The less electronegative atom has a partial positive charge due to the electron deficiency.
- The polarity of the bond is represented using an arrow pointing towards the more electronegative atom.
Examples of Polar Bonds:
1. In a water molecule (H2O), the oxygen atom is significantly more electronegative than the hydrogen atoms. Consequently, the oxygen attracts the shared electrons closer to itself, resulting in a partial negative charge on the oxygen and partial positive charges on the hydrogens.
2. In hydrochloric acid (HCl), the chlorine atom is more electronegative than the hydrogen atom, leading to a polar bond.
Nonpolar Bonds:
A nonpolar bond occurs when there is an equal sharing of electrons between two atoms due to similar or very close electronegativities. In this case, the electron density is evenly distributed across the bond, resulting in no distinct positive or negative charges on the atoms.
Key Points:
- Nonpolar bonds typically occur between atoms of the same element or elements with very similar electronegativities.
- The electronegativity difference between the atoms is usually less than 0.5.
Examples of Nonpolar Bonds:
1. In a diatomic molecule such as nitrogen (N2), both nitrogen atoms have the same electronegativity, resulting in a nonpolar bond.
2. Carbon dioxide (CO2) is another example of a nonpolar molecule. Although the carbon-oxygen bonds are polar, the symmetrical arrangement of the atoms cancels out the dipole moments, making the overall molecule nonpolar.
Conclusion:
In summary, the difference between polar and nonpolar bonds lies in the unequal or equal sharing of electrons, respectively. Polar bonds result from differences in electronegativity, leading to partial positive and negative charges on the atoms. Nonpolar bonds occur when electronegativity differences are minimal or nonexistent, resulting in an even distribution of electron density. Understanding these distinctions is crucial in predicting the behavior, solubility, and intermolecular forces of different compounds.
Introduction:
Molecular bonds play a fundamental role in the formation and stability of compounds. These bonds can be classified as either polar or nonpolar based on the electronegativity difference between the atoms involved. Understanding the difference between polar and nonpolar bonds is crucial in comprehending the behavior and properties of various substances.
Polar Bonds:
A polar bond occurs when there is an unequal sharing of electrons between two atoms due to differences in electronegativity. Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond. In a polar bond, one atom has a higher electronegativity than the other, resulting in an uneven distribution of electron density.
Key Points:
- The more electronegative atom attracts the shared electrons closer to its nucleus, creating a partial negative charge.
- The less electronegative atom has a partial positive charge due to the electron deficiency.
- The polarity of the bond is represented using an arrow pointing towards the more electronegative atom.
Examples of Polar Bonds:
1. In a water molecule (H2O), the oxygen atom is significantly more electronegative than the hydrogen atoms. Consequently, the oxygen attracts the shared electrons closer to itself, resulting in a partial negative charge on the oxygen and partial positive charges on the hydrogens.
2. In hydrochloric acid (HCl), the chlorine atom is more electronegative than the hydrogen atom, leading to a polar bond.
Nonpolar Bonds:
A nonpolar bond occurs when there is an equal sharing of electrons between two atoms due to similar or very close electronegativities. In this case, the electron density is evenly distributed across the bond, resulting in no distinct positive or negative charges on the atoms.
Key Points:
- Nonpolar bonds typically occur between atoms of the same element or elements with very similar electronegativities.
- The electronegativity difference between the atoms is usually less than 0.5.
Examples of Nonpolar Bonds:
1. In a diatomic molecule such as nitrogen (N2), both nitrogen atoms have the same electronegativity, resulting in a nonpolar bond.
2. Carbon dioxide (CO2) is another example of a nonpolar molecule. Although the carbon-oxygen bonds are polar, the symmetrical arrangement of the atoms cancels out the dipole moments, making the overall molecule nonpolar.
Conclusion:
In summary, the difference between polar and nonpolar bonds lies in the unequal or equal sharing of electrons, respectively. Polar bonds result from differences in electronegativity, leading to partial positive and negative charges on the atoms. Nonpolar bonds occur when electronegativity differences are minimal or nonexistent, resulting in an even distribution of electron density. Understanding these distinctions is crucial in predicting the behavior, solubility, and intermolecular forces of different compounds.
Attention Class 11 Students!
To make sure you are not studying endlessly, EduRev has designed Class 11 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 11.

|
Explore Courses for Class 11 exam
|

|
Similar Class 11 Doubts
What is the difference between polar and the non polar molecular bond
Question Description
What is the difference between polar and the non polar molecular bond for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about What is the difference between polar and the non polar molecular bond covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is the difference between polar and the non polar molecular bond.
What is the difference between polar and the non polar molecular bond for Class 11 2024 is part of Class 11 preparation. The Question and answers have been prepared according to the Class 11 exam syllabus. Information about What is the difference between polar and the non polar molecular bond covers all topics & solutions for Class 11 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is the difference between polar and the non polar molecular bond.
Solutions for What is the difference between polar and the non polar molecular bond in English & in Hindi are available as part of our courses for Class 11.
Download more important topics, notes, lectures and mock test series for Class 11 Exam by signing up for free.
Here you can find the meaning of What is the difference between polar and the non polar molecular bond defined & explained in the simplest way possible. Besides giving the explanation of
What is the difference between polar and the non polar molecular bond, a detailed solution for What is the difference between polar and the non polar molecular bond has been provided alongside types of What is the difference between polar and the non polar molecular bond theory, EduRev gives you an
ample number of questions to practice What is the difference between polar and the non polar molecular bond tests, examples and also practice Class 11 tests.

|
Explore Courses for Class 11 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























