ACT Exam > ACT Questions > The Ideal Gas Law is as follows:PV=nRTP is pr...
Start Learning for Free
The Ideal Gas Law is as follows:
PV=nRT
P is pressure as measured in Pascals, V is volume as measured in cubic meters, n is the number of moles of the gas, R is the gas constant known as 8.314 Joules per mole times Kelvin, and T is the temperature measured in Kelvin.
A class of students began studying the Ideal Gas Law and how the Pressure and the Volume relate to one another. They took 20 moles of a sample gas and kept the room at a temperature of 300 Kelvin. They then used different sized containers of the gas to limit and expand the volume. At each different volume, they measure the pressure of the gas on its container. The table they made from their results is seen in table 1.
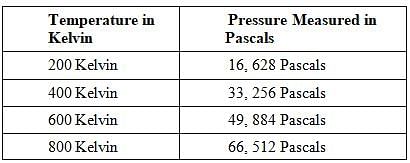
TABLE 1
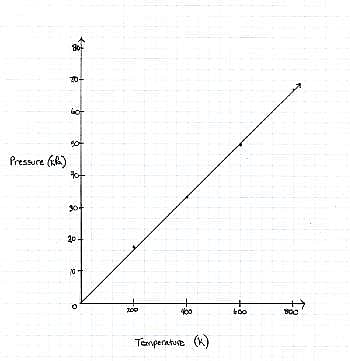
FIGURE 1
The graph the students made based on the data is seen in Figure 1.
Pressure is created by the movement of the gas molecules pushing against a container. 0 Kelvin is known as absolute 0, the temperature at which all molecule movement theoretically stops.
Q. Describe the relationship between the temperature and the pressure.
- a)They are inversely related. As temperature decreases, pressure increases.
- b)They are unrelated.
- c)They are directly related. As temperature increases, so does pressure.
- d)The pressure remains the same no matter the temperature.
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
The Ideal Gas Law is as follows:PV=nRTP is pressure as measured in Pas...
For every increase in temperature, there is a definite increase in pressure that can be found in the Ideal Gas Law equation. When the equation is solved for pressure
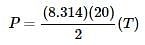
it can be thought of as the slope-intercept form of a line, with the y-intercept as 0 and the 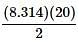 as the slope in front of the x-coordinate, which in this case is temperature.
as the slope in front of the x-coordinate, which in this case is temperature.
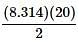 as the slope in front of the x-coordinate, which in this case is temperature.
as the slope in front of the x-coordinate, which in this case is temperature. Most Upvoted Answer
The Ideal Gas Law is as follows:PV=nRTP is pressure as measured in Pas...
For every increase in temperature, there is a definite increase in pressure that can be found in the Ideal Gas Law equation. When the equation is solved for pressure
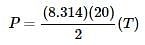
it can be thought of as the slope-intercept form of a line, with the y-intercept as 0 and the 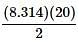 as the slope in front of the x-coordinate, which in this case is temperature.
as the slope in front of the x-coordinate, which in this case is temperature.
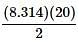 as the slope in front of the x-coordinate, which in this case is temperature.
as the slope in front of the x-coordinate, which in this case is temperature.

|
Explore Courses for ACT exam
|

|
Similar ACT Doubts
The Ideal Gas Law is as follows:PV=nRTP is pressure as measured in Pascals, V is volume as measured in cubic meters, n is the number of moles of the gas, R is the gas constant known as 8.314 Joules per mole times Kelvin, and T is the temperature measured in Kelvin.A class of students began studying the Ideal Gas Law and how the Pressure and the Volume relate to one another. They took 20 moles of a sample gas and kept the room at a temperature of 300 Kelvin. They then used different sized containers of the gas to limit and expand the volume. At each different volume, they measure the pressure of the gas on its container. The table they made from their results is seen in table 1.TABLE 1FIGURE 1The graph the students made based on the data is seen in Figure 1.Pressure is created by the movement of the gas molecules pushing against a container. 0 Kelvin is known as absolute 0, the temperature at which all molecule movement theoretically stops.Q. Describe the relationship between the temperature and the pressure.a)They are inversely related. As temperature decreases, pressure increases.b)They are unrelated.c)They are directly related. As temperature increases, so does pressure. d)The pressure remains the same no matter the temperature. Correct answer is option 'C'. Can you explain this answer?
Question Description
The Ideal Gas Law is as follows:PV=nRTP is pressure as measured in Pascals, V is volume as measured in cubic meters, n is the number of moles of the gas, R is the gas constant known as 8.314 Joules per mole times Kelvin, and T is the temperature measured in Kelvin.A class of students began studying the Ideal Gas Law and how the Pressure and the Volume relate to one another. They took 20 moles of a sample gas and kept the room at a temperature of 300 Kelvin. They then used different sized containers of the gas to limit and expand the volume. At each different volume, they measure the pressure of the gas on its container. The table they made from their results is seen in table 1.TABLE 1FIGURE 1The graph the students made based on the data is seen in Figure 1.Pressure is created by the movement of the gas molecules pushing against a container. 0 Kelvin is known as absolute 0, the temperature at which all molecule movement theoretically stops.Q. Describe the relationship between the temperature and the pressure.a)They are inversely related. As temperature decreases, pressure increases.b)They are unrelated.c)They are directly related. As temperature increases, so does pressure. d)The pressure remains the same no matter the temperature. Correct answer is option 'C'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about The Ideal Gas Law is as follows:PV=nRTP is pressure as measured in Pascals, V is volume as measured in cubic meters, n is the number of moles of the gas, R is the gas constant known as 8.314 Joules per mole times Kelvin, and T is the temperature measured in Kelvin.A class of students began studying the Ideal Gas Law and how the Pressure and the Volume relate to one another. They took 20 moles of a sample gas and kept the room at a temperature of 300 Kelvin. They then used different sized containers of the gas to limit and expand the volume. At each different volume, they measure the pressure of the gas on its container. The table they made from their results is seen in table 1.TABLE 1FIGURE 1The graph the students made based on the data is seen in Figure 1.Pressure is created by the movement of the gas molecules pushing against a container. 0 Kelvin is known as absolute 0, the temperature at which all molecule movement theoretically stops.Q. Describe the relationship between the temperature and the pressure.a)They are inversely related. As temperature decreases, pressure increases.b)They are unrelated.c)They are directly related. As temperature increases, so does pressure. d)The pressure remains the same no matter the temperature. Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The Ideal Gas Law is as follows:PV=nRTP is pressure as measured in Pascals, V is volume as measured in cubic meters, n is the number of moles of the gas, R is the gas constant known as 8.314 Joules per mole times Kelvin, and T is the temperature measured in Kelvin.A class of students began studying the Ideal Gas Law and how the Pressure and the Volume relate to one another. They took 20 moles of a sample gas and kept the room at a temperature of 300 Kelvin. They then used different sized containers of the gas to limit and expand the volume. At each different volume, they measure the pressure of the gas on its container. The table they made from their results is seen in table 1.TABLE 1FIGURE 1The graph the students made based on the data is seen in Figure 1.Pressure is created by the movement of the gas molecules pushing against a container. 0 Kelvin is known as absolute 0, the temperature at which all molecule movement theoretically stops.Q. Describe the relationship between the temperature and the pressure.a)They are inversely related. As temperature decreases, pressure increases.b)They are unrelated.c)They are directly related. As temperature increases, so does pressure. d)The pressure remains the same no matter the temperature. Correct answer is option 'C'. Can you explain this answer?.
The Ideal Gas Law is as follows:PV=nRTP is pressure as measured in Pascals, V is volume as measured in cubic meters, n is the number of moles of the gas, R is the gas constant known as 8.314 Joules per mole times Kelvin, and T is the temperature measured in Kelvin.A class of students began studying the Ideal Gas Law and how the Pressure and the Volume relate to one another. They took 20 moles of a sample gas and kept the room at a temperature of 300 Kelvin. They then used different sized containers of the gas to limit and expand the volume. At each different volume, they measure the pressure of the gas on its container. The table they made from their results is seen in table 1.TABLE 1FIGURE 1The graph the students made based on the data is seen in Figure 1.Pressure is created by the movement of the gas molecules pushing against a container. 0 Kelvin is known as absolute 0, the temperature at which all molecule movement theoretically stops.Q. Describe the relationship between the temperature and the pressure.a)They are inversely related. As temperature decreases, pressure increases.b)They are unrelated.c)They are directly related. As temperature increases, so does pressure. d)The pressure remains the same no matter the temperature. Correct answer is option 'C'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about The Ideal Gas Law is as follows:PV=nRTP is pressure as measured in Pascals, V is volume as measured in cubic meters, n is the number of moles of the gas, R is the gas constant known as 8.314 Joules per mole times Kelvin, and T is the temperature measured in Kelvin.A class of students began studying the Ideal Gas Law and how the Pressure and the Volume relate to one another. They took 20 moles of a sample gas and kept the room at a temperature of 300 Kelvin. They then used different sized containers of the gas to limit and expand the volume. At each different volume, they measure the pressure of the gas on its container. The table they made from their results is seen in table 1.TABLE 1FIGURE 1The graph the students made based on the data is seen in Figure 1.Pressure is created by the movement of the gas molecules pushing against a container. 0 Kelvin is known as absolute 0, the temperature at which all molecule movement theoretically stops.Q. Describe the relationship between the temperature and the pressure.a)They are inversely related. As temperature decreases, pressure increases.b)They are unrelated.c)They are directly related. As temperature increases, so does pressure. d)The pressure remains the same no matter the temperature. Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The Ideal Gas Law is as follows:PV=nRTP is pressure as measured in Pascals, V is volume as measured in cubic meters, n is the number of moles of the gas, R is the gas constant known as 8.314 Joules per mole times Kelvin, and T is the temperature measured in Kelvin.A class of students began studying the Ideal Gas Law and how the Pressure and the Volume relate to one another. They took 20 moles of a sample gas and kept the room at a temperature of 300 Kelvin. They then used different sized containers of the gas to limit and expand the volume. At each different volume, they measure the pressure of the gas on its container. The table they made from their results is seen in table 1.TABLE 1FIGURE 1The graph the students made based on the data is seen in Figure 1.Pressure is created by the movement of the gas molecules pushing against a container. 0 Kelvin is known as absolute 0, the temperature at which all molecule movement theoretically stops.Q. Describe the relationship between the temperature and the pressure.a)They are inversely related. As temperature decreases, pressure increases.b)They are unrelated.c)They are directly related. As temperature increases, so does pressure. d)The pressure remains the same no matter the temperature. Correct answer is option 'C'. Can you explain this answer?.
Solutions for The Ideal Gas Law is as follows:PV=nRTP is pressure as measured in Pascals, V is volume as measured in cubic meters, n is the number of moles of the gas, R is the gas constant known as 8.314 Joules per mole times Kelvin, and T is the temperature measured in Kelvin.A class of students began studying the Ideal Gas Law and how the Pressure and the Volume relate to one another. They took 20 moles of a sample gas and kept the room at a temperature of 300 Kelvin. They then used different sized containers of the gas to limit and expand the volume. At each different volume, they measure the pressure of the gas on its container. The table they made from their results is seen in table 1.TABLE 1FIGURE 1The graph the students made based on the data is seen in Figure 1.Pressure is created by the movement of the gas molecules pushing against a container. 0 Kelvin is known as absolute 0, the temperature at which all molecule movement theoretically stops.Q. Describe the relationship between the temperature and the pressure.a)They are inversely related. As temperature decreases, pressure increases.b)They are unrelated.c)They are directly related. As temperature increases, so does pressure. d)The pressure remains the same no matter the temperature. Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for ACT.
Download more important topics, notes, lectures and mock test series for ACT Exam by signing up for free.
Here you can find the meaning of The Ideal Gas Law is as follows:PV=nRTP is pressure as measured in Pascals, V is volume as measured in cubic meters, n is the number of moles of the gas, R is the gas constant known as 8.314 Joules per mole times Kelvin, and T is the temperature measured in Kelvin.A class of students began studying the Ideal Gas Law and how the Pressure and the Volume relate to one another. They took 20 moles of a sample gas and kept the room at a temperature of 300 Kelvin. They then used different sized containers of the gas to limit and expand the volume. At each different volume, they measure the pressure of the gas on its container. The table they made from their results is seen in table 1.TABLE 1FIGURE 1The graph the students made based on the data is seen in Figure 1.Pressure is created by the movement of the gas molecules pushing against a container. 0 Kelvin is known as absolute 0, the temperature at which all molecule movement theoretically stops.Q. Describe the relationship between the temperature and the pressure.a)They are inversely related. As temperature decreases, pressure increases.b)They are unrelated.c)They are directly related. As temperature increases, so does pressure. d)The pressure remains the same no matter the temperature. Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The Ideal Gas Law is as follows:PV=nRTP is pressure as measured in Pascals, V is volume as measured in cubic meters, n is the number of moles of the gas, R is the gas constant known as 8.314 Joules per mole times Kelvin, and T is the temperature measured in Kelvin.A class of students began studying the Ideal Gas Law and how the Pressure and the Volume relate to one another. They took 20 moles of a sample gas and kept the room at a temperature of 300 Kelvin. They then used different sized containers of the gas to limit and expand the volume. At each different volume, they measure the pressure of the gas on its container. The table they made from their results is seen in table 1.TABLE 1FIGURE 1The graph the students made based on the data is seen in Figure 1.Pressure is created by the movement of the gas molecules pushing against a container. 0 Kelvin is known as absolute 0, the temperature at which all molecule movement theoretically stops.Q. Describe the relationship between the temperature and the pressure.a)They are inversely related. As temperature decreases, pressure increases.b)They are unrelated.c)They are directly related. As temperature increases, so does pressure. d)The pressure remains the same no matter the temperature. Correct answer is option 'C'. Can you explain this answer?, a detailed solution for The Ideal Gas Law is as follows:PV=nRTP is pressure as measured in Pascals, V is volume as measured in cubic meters, n is the number of moles of the gas, R is the gas constant known as 8.314 Joules per mole times Kelvin, and T is the temperature measured in Kelvin.A class of students began studying the Ideal Gas Law and how the Pressure and the Volume relate to one another. They took 20 moles of a sample gas and kept the room at a temperature of 300 Kelvin. They then used different sized containers of the gas to limit and expand the volume. At each different volume, they measure the pressure of the gas on its container. The table they made from their results is seen in table 1.TABLE 1FIGURE 1The graph the students made based on the data is seen in Figure 1.Pressure is created by the movement of the gas molecules pushing against a container. 0 Kelvin is known as absolute 0, the temperature at which all molecule movement theoretically stops.Q. Describe the relationship between the temperature and the pressure.a)They are inversely related. As temperature decreases, pressure increases.b)They are unrelated.c)They are directly related. As temperature increases, so does pressure. d)The pressure remains the same no matter the temperature. Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of The Ideal Gas Law is as follows:PV=nRTP is pressure as measured in Pascals, V is volume as measured in cubic meters, n is the number of moles of the gas, R is the gas constant known as 8.314 Joules per mole times Kelvin, and T is the temperature measured in Kelvin.A class of students began studying the Ideal Gas Law and how the Pressure and the Volume relate to one another. They took 20 moles of a sample gas and kept the room at a temperature of 300 Kelvin. They then used different sized containers of the gas to limit and expand the volume. At each different volume, they measure the pressure of the gas on its container. The table they made from their results is seen in table 1.TABLE 1FIGURE 1The graph the students made based on the data is seen in Figure 1.Pressure is created by the movement of the gas molecules pushing against a container. 0 Kelvin is known as absolute 0, the temperature at which all molecule movement theoretically stops.Q. Describe the relationship between the temperature and the pressure.a)They are inversely related. As temperature decreases, pressure increases.b)They are unrelated.c)They are directly related. As temperature increases, so does pressure. d)The pressure remains the same no matter the temperature. Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The Ideal Gas Law is as follows:PV=nRTP is pressure as measured in Pascals, V is volume as measured in cubic meters, n is the number of moles of the gas, R is the gas constant known as 8.314 Joules per mole times Kelvin, and T is the temperature measured in Kelvin.A class of students began studying the Ideal Gas Law and how the Pressure and the Volume relate to one another. They took 20 moles of a sample gas and kept the room at a temperature of 300 Kelvin. They then used different sized containers of the gas to limit and expand the volume. At each different volume, they measure the pressure of the gas on its container. The table they made from their results is seen in table 1.TABLE 1FIGURE 1The graph the students made based on the data is seen in Figure 1.Pressure is created by the movement of the gas molecules pushing against a container. 0 Kelvin is known as absolute 0, the temperature at which all molecule movement theoretically stops.Q. Describe the relationship between the temperature and the pressure.a)They are inversely related. As temperature decreases, pressure increases.b)They are unrelated.c)They are directly related. As temperature increases, so does pressure. d)The pressure remains the same no matter the temperature. Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice ACT tests.

|
Explore Courses for ACT exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























