ACT Exam > ACT Questions > Two scientists wanted to test the solubility ...
Start Learning for Free
Two scientists wanted to test the solubility of different substances. Solubility is a measure of how many moles of a given substance (known as the solute) can dissolve in a given volume of another substance (known as the solvent). The solvent can also be thought of as the substance present in greater amount, while the solute can be seen as the substance present in lesser amount. The scientists performed the following experiments to investigate this property.
Experiment 1
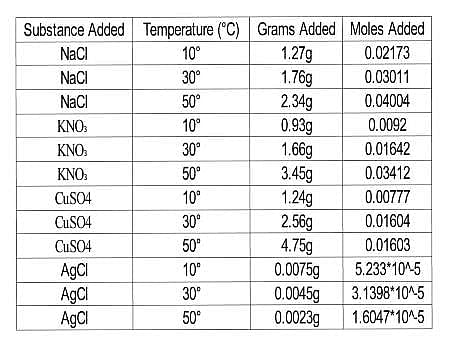
The scientists tested the number of moles of several substances that could be completely dissolved in 50mL of water at various temperatures. They made their solutions by slowly adding amounts of each substance to beakers sitting on a hot plate containing water and a stirring rod until no more of the substance dissolved in the solution. The beakers were weighed before and after the additions and the difference in mass was calculated to be the added mass of the substance. The researchers then calculated the number of moles that dissolved for each trial using the molecular mass and the recorded mass for each trial. Results are recorded in Table 1.
Table 1
Experiment 2
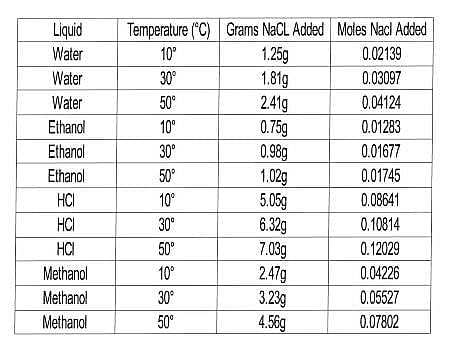
In this experiment, the scientists wanted to test the solubility of NaCl in a variety of liquids at several temperatures. Their procedure was similar to that of Experiment 1, but with a range of liquids and only one solid. The results are compiled in Table 2.
Table 2
Q. Which of the following correctly ranks the solutes from Experiment 1 in decreasing order of solubility in water at 50∘C?
- a)NaCl,KNO3,CuSO4,AgCl
- b)CuSO4,KNO3,NaCl,AgCl
- c)CuSO4,NaCl,KNO3,AgCl
- d)AgCl,NaCl,KNO3,CuSO4
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
Two scientists wanted to test the solubility of different substances. ...
The introduction explains that solubility is a measure of how many moles of a substance can dissolve in a given volume of another substance. This is a very important clue that tells us we need to focus on the "moles added" column rather than the "grams added" column. We need to rank our substances in decreasing order, so we need start with the one with the highest solubility at 50∘C.
The number of moles dissolved at 50∘C are as follows.
NaCl: 0.04004
KNO3: 0.03412
CuSO4: 0.1603
AgCl: 1.6047∗10−5
So the correct order is:
NaCl,KNO3,CuSO4,AgCl
Most Upvoted Answer
Two scientists wanted to test the solubility of different substances. ...
The introduction explains that solubility is a measure of how many moles of a substance can dissolve in a given volume of another substance. This is a very important clue that tells us we need to focus on the "moles added" column rather than the "grams added" column. We need to rank our substances in decreasing order, so we need start with the one with the highest solubility at 50∘C.
The number of moles dissolved at 50∘C are as follows.
NaCl: 0.04004
KNO3: 0.03412
CuSO4: 0.1603
AgCl: 1.6047∗10−5
So the correct order is:
NaCl,KNO3,CuSO4,AgCl

|
Explore Courses for ACT exam
|

|
Similar ACT Doubts
Two scientists wanted to test the solubility of different substances. Solubility is a measure of how many moles of a given substance (known as the solute) can dissolve in a given volume of another substance (known as the solvent). The solvent can also be thought of as the substance present in greater amount, while the solute can be seen as the substance present in lesser amount. The scientists performed the following experiments to investigate this property.Experiment 1The scientists tested the number of moles of several substances that could be completely dissolved in 50mL of water at various temperatures. They made their solutions by slowly adding amounts of each substance to beakers sitting on a hot plate containing water and a stirring rod until no more of the substance dissolved in the solution. The beakers were weighed before and after the additions and the difference in mass was calculated to be the added mass of the substance. The researchers then calculated the number of moles that dissolved for each trial using the molecular mass and the recorded mass for each trial. Results are recorded in Table 1.Table 1Experiment 2In this experiment, the scientists wanted to test the solubility of NaCl in a variety of liquids at several temperatures. Their procedure was similar to that of Experiment 1, but with a range of liquids and only one solid. The results are compiled in Table 2.Table 2Q.Which of the following correctly ranks the solutes from Experiment 1 in decreasing order of solubility in water at 50C?a)NaCl,KNO3,CuSO4,AgClb)CuSO4,KNO3,NaCl,AgClc)CuSO4,NaCl,KNO3,AgCld)AgCl,NaCl,KNO3,CuSO4Correct answer is option 'A'. Can you explain this answer?
Question Description
Two scientists wanted to test the solubility of different substances. Solubility is a measure of how many moles of a given substance (known as the solute) can dissolve in a given volume of another substance (known as the solvent). The solvent can also be thought of as the substance present in greater amount, while the solute can be seen as the substance present in lesser amount. The scientists performed the following experiments to investigate this property.Experiment 1The scientists tested the number of moles of several substances that could be completely dissolved in 50mL of water at various temperatures. They made their solutions by slowly adding amounts of each substance to beakers sitting on a hot plate containing water and a stirring rod until no more of the substance dissolved in the solution. The beakers were weighed before and after the additions and the difference in mass was calculated to be the added mass of the substance. The researchers then calculated the number of moles that dissolved for each trial using the molecular mass and the recorded mass for each trial. Results are recorded in Table 1.Table 1Experiment 2In this experiment, the scientists wanted to test the solubility of NaCl in a variety of liquids at several temperatures. Their procedure was similar to that of Experiment 1, but with a range of liquids and only one solid. The results are compiled in Table 2.Table 2Q.Which of the following correctly ranks the solutes from Experiment 1 in decreasing order of solubility in water at 50C?a)NaCl,KNO3,CuSO4,AgClb)CuSO4,KNO3,NaCl,AgClc)CuSO4,NaCl,KNO3,AgCld)AgCl,NaCl,KNO3,CuSO4Correct answer is option 'A'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Two scientists wanted to test the solubility of different substances. Solubility is a measure of how many moles of a given substance (known as the solute) can dissolve in a given volume of another substance (known as the solvent). The solvent can also be thought of as the substance present in greater amount, while the solute can be seen as the substance present in lesser amount. The scientists performed the following experiments to investigate this property.Experiment 1The scientists tested the number of moles of several substances that could be completely dissolved in 50mL of water at various temperatures. They made their solutions by slowly adding amounts of each substance to beakers sitting on a hot plate containing water and a stirring rod until no more of the substance dissolved in the solution. The beakers were weighed before and after the additions and the difference in mass was calculated to be the added mass of the substance. The researchers then calculated the number of moles that dissolved for each trial using the molecular mass and the recorded mass for each trial. Results are recorded in Table 1.Table 1Experiment 2In this experiment, the scientists wanted to test the solubility of NaCl in a variety of liquids at several temperatures. Their procedure was similar to that of Experiment 1, but with a range of liquids and only one solid. The results are compiled in Table 2.Table 2Q.Which of the following correctly ranks the solutes from Experiment 1 in decreasing order of solubility in water at 50C?a)NaCl,KNO3,CuSO4,AgClb)CuSO4,KNO3,NaCl,AgClc)CuSO4,NaCl,KNO3,AgCld)AgCl,NaCl,KNO3,CuSO4Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Two scientists wanted to test the solubility of different substances. Solubility is a measure of how many moles of a given substance (known as the solute) can dissolve in a given volume of another substance (known as the solvent). The solvent can also be thought of as the substance present in greater amount, while the solute can be seen as the substance present in lesser amount. The scientists performed the following experiments to investigate this property.Experiment 1The scientists tested the number of moles of several substances that could be completely dissolved in 50mL of water at various temperatures. They made their solutions by slowly adding amounts of each substance to beakers sitting on a hot plate containing water and a stirring rod until no more of the substance dissolved in the solution. The beakers were weighed before and after the additions and the difference in mass was calculated to be the added mass of the substance. The researchers then calculated the number of moles that dissolved for each trial using the molecular mass and the recorded mass for each trial. Results are recorded in Table 1.Table 1Experiment 2In this experiment, the scientists wanted to test the solubility of NaCl in a variety of liquids at several temperatures. Their procedure was similar to that of Experiment 1, but with a range of liquids and only one solid. The results are compiled in Table 2.Table 2Q.Which of the following correctly ranks the solutes from Experiment 1 in decreasing order of solubility in water at 50C?a)NaCl,KNO3,CuSO4,AgClb)CuSO4,KNO3,NaCl,AgClc)CuSO4,NaCl,KNO3,AgCld)AgCl,NaCl,KNO3,CuSO4Correct answer is option 'A'. Can you explain this answer?.
Two scientists wanted to test the solubility of different substances. Solubility is a measure of how many moles of a given substance (known as the solute) can dissolve in a given volume of another substance (known as the solvent). The solvent can also be thought of as the substance present in greater amount, while the solute can be seen as the substance present in lesser amount. The scientists performed the following experiments to investigate this property.Experiment 1The scientists tested the number of moles of several substances that could be completely dissolved in 50mL of water at various temperatures. They made their solutions by slowly adding amounts of each substance to beakers sitting on a hot plate containing water and a stirring rod until no more of the substance dissolved in the solution. The beakers were weighed before and after the additions and the difference in mass was calculated to be the added mass of the substance. The researchers then calculated the number of moles that dissolved for each trial using the molecular mass and the recorded mass for each trial. Results are recorded in Table 1.Table 1Experiment 2In this experiment, the scientists wanted to test the solubility of NaCl in a variety of liquids at several temperatures. Their procedure was similar to that of Experiment 1, but with a range of liquids and only one solid. The results are compiled in Table 2.Table 2Q.Which of the following correctly ranks the solutes from Experiment 1 in decreasing order of solubility in water at 50C?a)NaCl,KNO3,CuSO4,AgClb)CuSO4,KNO3,NaCl,AgClc)CuSO4,NaCl,KNO3,AgCld)AgCl,NaCl,KNO3,CuSO4Correct answer is option 'A'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Two scientists wanted to test the solubility of different substances. Solubility is a measure of how many moles of a given substance (known as the solute) can dissolve in a given volume of another substance (known as the solvent). The solvent can also be thought of as the substance present in greater amount, while the solute can be seen as the substance present in lesser amount. The scientists performed the following experiments to investigate this property.Experiment 1The scientists tested the number of moles of several substances that could be completely dissolved in 50mL of water at various temperatures. They made their solutions by slowly adding amounts of each substance to beakers sitting on a hot plate containing water and a stirring rod until no more of the substance dissolved in the solution. The beakers were weighed before and after the additions and the difference in mass was calculated to be the added mass of the substance. The researchers then calculated the number of moles that dissolved for each trial using the molecular mass and the recorded mass for each trial. Results are recorded in Table 1.Table 1Experiment 2In this experiment, the scientists wanted to test the solubility of NaCl in a variety of liquids at several temperatures. Their procedure was similar to that of Experiment 1, but with a range of liquids and only one solid. The results are compiled in Table 2.Table 2Q.Which of the following correctly ranks the solutes from Experiment 1 in decreasing order of solubility in water at 50C?a)NaCl,KNO3,CuSO4,AgClb)CuSO4,KNO3,NaCl,AgClc)CuSO4,NaCl,KNO3,AgCld)AgCl,NaCl,KNO3,CuSO4Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Two scientists wanted to test the solubility of different substances. Solubility is a measure of how many moles of a given substance (known as the solute) can dissolve in a given volume of another substance (known as the solvent). The solvent can also be thought of as the substance present in greater amount, while the solute can be seen as the substance present in lesser amount. The scientists performed the following experiments to investigate this property.Experiment 1The scientists tested the number of moles of several substances that could be completely dissolved in 50mL of water at various temperatures. They made their solutions by slowly adding amounts of each substance to beakers sitting on a hot plate containing water and a stirring rod until no more of the substance dissolved in the solution. The beakers were weighed before and after the additions and the difference in mass was calculated to be the added mass of the substance. The researchers then calculated the number of moles that dissolved for each trial using the molecular mass and the recorded mass for each trial. Results are recorded in Table 1.Table 1Experiment 2In this experiment, the scientists wanted to test the solubility of NaCl in a variety of liquids at several temperatures. Their procedure was similar to that of Experiment 1, but with a range of liquids and only one solid. The results are compiled in Table 2.Table 2Q.Which of the following correctly ranks the solutes from Experiment 1 in decreasing order of solubility in water at 50C?a)NaCl,KNO3,CuSO4,AgClb)CuSO4,KNO3,NaCl,AgClc)CuSO4,NaCl,KNO3,AgCld)AgCl,NaCl,KNO3,CuSO4Correct answer is option 'A'. Can you explain this answer?.
Solutions for Two scientists wanted to test the solubility of different substances. Solubility is a measure of how many moles of a given substance (known as the solute) can dissolve in a given volume of another substance (known as the solvent). The solvent can also be thought of as the substance present in greater amount, while the solute can be seen as the substance present in lesser amount. The scientists performed the following experiments to investigate this property.Experiment 1The scientists tested the number of moles of several substances that could be completely dissolved in 50mL of water at various temperatures. They made their solutions by slowly adding amounts of each substance to beakers sitting on a hot plate containing water and a stirring rod until no more of the substance dissolved in the solution. The beakers were weighed before and after the additions and the difference in mass was calculated to be the added mass of the substance. The researchers then calculated the number of moles that dissolved for each trial using the molecular mass and the recorded mass for each trial. Results are recorded in Table 1.Table 1Experiment 2In this experiment, the scientists wanted to test the solubility of NaCl in a variety of liquids at several temperatures. Their procedure was similar to that of Experiment 1, but with a range of liquids and only one solid. The results are compiled in Table 2.Table 2Q.Which of the following correctly ranks the solutes from Experiment 1 in decreasing order of solubility in water at 50C?a)NaCl,KNO3,CuSO4,AgClb)CuSO4,KNO3,NaCl,AgClc)CuSO4,NaCl,KNO3,AgCld)AgCl,NaCl,KNO3,CuSO4Correct answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for ACT.
Download more important topics, notes, lectures and mock test series for ACT Exam by signing up for free.
Here you can find the meaning of Two scientists wanted to test the solubility of different substances. Solubility is a measure of how many moles of a given substance (known as the solute) can dissolve in a given volume of another substance (known as the solvent). The solvent can also be thought of as the substance present in greater amount, while the solute can be seen as the substance present in lesser amount. The scientists performed the following experiments to investigate this property.Experiment 1The scientists tested the number of moles of several substances that could be completely dissolved in 50mL of water at various temperatures. They made their solutions by slowly adding amounts of each substance to beakers sitting on a hot plate containing water and a stirring rod until no more of the substance dissolved in the solution. The beakers were weighed before and after the additions and the difference in mass was calculated to be the added mass of the substance. The researchers then calculated the number of moles that dissolved for each trial using the molecular mass and the recorded mass for each trial. Results are recorded in Table 1.Table 1Experiment 2In this experiment, the scientists wanted to test the solubility of NaCl in a variety of liquids at several temperatures. Their procedure was similar to that of Experiment 1, but with a range of liquids and only one solid. The results are compiled in Table 2.Table 2Q.Which of the following correctly ranks the solutes from Experiment 1 in decreasing order of solubility in water at 50C?a)NaCl,KNO3,CuSO4,AgClb)CuSO4,KNO3,NaCl,AgClc)CuSO4,NaCl,KNO3,AgCld)AgCl,NaCl,KNO3,CuSO4Correct answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Two scientists wanted to test the solubility of different substances. Solubility is a measure of how many moles of a given substance (known as the solute) can dissolve in a given volume of another substance (known as the solvent). The solvent can also be thought of as the substance present in greater amount, while the solute can be seen as the substance present in lesser amount. The scientists performed the following experiments to investigate this property.Experiment 1The scientists tested the number of moles of several substances that could be completely dissolved in 50mL of water at various temperatures. They made their solutions by slowly adding amounts of each substance to beakers sitting on a hot plate containing water and a stirring rod until no more of the substance dissolved in the solution. The beakers were weighed before and after the additions and the difference in mass was calculated to be the added mass of the substance. The researchers then calculated the number of moles that dissolved for each trial using the molecular mass and the recorded mass for each trial. Results are recorded in Table 1.Table 1Experiment 2In this experiment, the scientists wanted to test the solubility of NaCl in a variety of liquids at several temperatures. Their procedure was similar to that of Experiment 1, but with a range of liquids and only one solid. The results are compiled in Table 2.Table 2Q.Which of the following correctly ranks the solutes from Experiment 1 in decreasing order of solubility in water at 50C?a)NaCl,KNO3,CuSO4,AgClb)CuSO4,KNO3,NaCl,AgClc)CuSO4,NaCl,KNO3,AgCld)AgCl,NaCl,KNO3,CuSO4Correct answer is option 'A'. Can you explain this answer?, a detailed solution for Two scientists wanted to test the solubility of different substances. Solubility is a measure of how many moles of a given substance (known as the solute) can dissolve in a given volume of another substance (known as the solvent). The solvent can also be thought of as the substance present in greater amount, while the solute can be seen as the substance present in lesser amount. The scientists performed the following experiments to investigate this property.Experiment 1The scientists tested the number of moles of several substances that could be completely dissolved in 50mL of water at various temperatures. They made their solutions by slowly adding amounts of each substance to beakers sitting on a hot plate containing water and a stirring rod until no more of the substance dissolved in the solution. The beakers were weighed before and after the additions and the difference in mass was calculated to be the added mass of the substance. The researchers then calculated the number of moles that dissolved for each trial using the molecular mass and the recorded mass for each trial. Results are recorded in Table 1.Table 1Experiment 2In this experiment, the scientists wanted to test the solubility of NaCl in a variety of liquids at several temperatures. Their procedure was similar to that of Experiment 1, but with a range of liquids and only one solid. The results are compiled in Table 2.Table 2Q.Which of the following correctly ranks the solutes from Experiment 1 in decreasing order of solubility in water at 50C?a)NaCl,KNO3,CuSO4,AgClb)CuSO4,KNO3,NaCl,AgClc)CuSO4,NaCl,KNO3,AgCld)AgCl,NaCl,KNO3,CuSO4Correct answer is option 'A'. Can you explain this answer? has been provided alongside types of Two scientists wanted to test the solubility of different substances. Solubility is a measure of how many moles of a given substance (known as the solute) can dissolve in a given volume of another substance (known as the solvent). The solvent can also be thought of as the substance present in greater amount, while the solute can be seen as the substance present in lesser amount. The scientists performed the following experiments to investigate this property.Experiment 1The scientists tested the number of moles of several substances that could be completely dissolved in 50mL of water at various temperatures. They made their solutions by slowly adding amounts of each substance to beakers sitting on a hot plate containing water and a stirring rod until no more of the substance dissolved in the solution. The beakers were weighed before and after the additions and the difference in mass was calculated to be the added mass of the substance. The researchers then calculated the number of moles that dissolved for each trial using the molecular mass and the recorded mass for each trial. Results are recorded in Table 1.Table 1Experiment 2In this experiment, the scientists wanted to test the solubility of NaCl in a variety of liquids at several temperatures. Their procedure was similar to that of Experiment 1, but with a range of liquids and only one solid. The results are compiled in Table 2.Table 2Q.Which of the following correctly ranks the solutes from Experiment 1 in decreasing order of solubility in water at 50C?a)NaCl,KNO3,CuSO4,AgClb)CuSO4,KNO3,NaCl,AgClc)CuSO4,NaCl,KNO3,AgCld)AgCl,NaCl,KNO3,CuSO4Correct answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Two scientists wanted to test the solubility of different substances. Solubility is a measure of how many moles of a given substance (known as the solute) can dissolve in a given volume of another substance (known as the solvent). The solvent can also be thought of as the substance present in greater amount, while the solute can be seen as the substance present in lesser amount. The scientists performed the following experiments to investigate this property.Experiment 1The scientists tested the number of moles of several substances that could be completely dissolved in 50mL of water at various temperatures. They made their solutions by slowly adding amounts of each substance to beakers sitting on a hot plate containing water and a stirring rod until no more of the substance dissolved in the solution. The beakers were weighed before and after the additions and the difference in mass was calculated to be the added mass of the substance. The researchers then calculated the number of moles that dissolved for each trial using the molecular mass and the recorded mass for each trial. Results are recorded in Table 1.Table 1Experiment 2In this experiment, the scientists wanted to test the solubility of NaCl in a variety of liquids at several temperatures. Their procedure was similar to that of Experiment 1, but with a range of liquids and only one solid. The results are compiled in Table 2.Table 2Q.Which of the following correctly ranks the solutes from Experiment 1 in decreasing order of solubility in water at 50C?a)NaCl,KNO3,CuSO4,AgClb)CuSO4,KNO3,NaCl,AgClc)CuSO4,NaCl,KNO3,AgCld)AgCl,NaCl,KNO3,CuSO4Correct answer is option 'A'. Can you explain this answer? tests, examples and also practice ACT tests.

|
Explore Courses for ACT exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























