ACT Exam > ACT Questions > A new drug is in its clinical trial phase. Th...
Start Learning for Free
A new drug is in its clinical trial phase. The graph below shows the data for participants sleep patterns before medication use and then after using the medication for three months. Series 1 shows the average hours slept a night for each participant prior to medication. Series 2 shows the average hours slept a night for each participant after three months of use. The average hours slept a night was obtained by recording sleep every night for one month, and then finding the average. Patients one through five took a placebo pil, not the actual Moonlight medication. Note: The y-axis is measured in hours slept a night, while the x-axis lists each anonymous patient numerically.
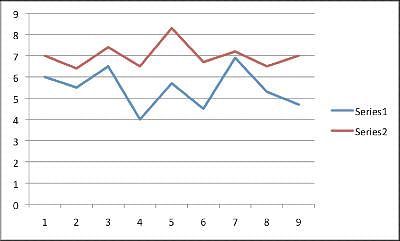
Q. The overall trend when comparing sleep before and after mediciation use is __________.
- a)There was not a difference in the average hours slept a night by a patient before the medication use and after the medication use.
- b)Patients who slept the most prior to the medication saw the least increase in hours slept when on the medication.
- c)Patients who slept the most prior to the medication saw the greatest increase in hours slept when on the medication.
- d)Patients who slept the least prior to the medication saw the greatest increase in hours slept when on the medication.
- e)All patients experienced an increase in hours slept when on the medication, except those taking the placebo.
Correct answer is option 'D'. Can you explain this answer?
Most Upvoted Answer
A new drug is in its clinical trial phase. The graph below shows the d...
Looking at the graph, one will see the patients who slept the least before medication usage (patients 4, 6, and 9), had the largest increase in hours slept. Further, patient 7 and patient 3 had the greatest amount of hours slept prior to medication usage. These two patients had the smallest increase in average sleeping hours.

|
Explore Courses for ACT exam
|

|
Similar ACT Doubts
A new drug is in its clinical trial phase. The graph below shows the data for participants sleep patterns before medication use and then after using the medication for three months. Series 1 shows the average hours slept a night for each participant prior to medication. Series 2 shows the average hours slept a night for each participant after three months of use. The average hours slept a night was obtained by recording sleep every night for one month, and then finding the average. Patients one through five took a placebo pil, not the actual Moonlight medication. Note: The y-axis is measured in hours slept a night, while the x-axis lists each anonymous patient numerically.Q.The overall trend when comparing sleep before and after mediciation use is __________.a)There was not a difference in the average hours slept a night by a patient before the medication use and after the medication use.b)Patients who slept the most prior to the medication saw the least increase in hours slept when on the medication.c)Patients who slept the most prior to the medication saw the greatest increase in hours slept when on the medication.d)Patients who slept the least prior to the medication saw the greatest increase in hours slept when on the medication.e)All patients experienced an increase in hours slept when on the medication, except those taking the placebo.Correct answer is option 'D'. Can you explain this answer?
Question Description
A new drug is in its clinical trial phase. The graph below shows the data for participants sleep patterns before medication use and then after using the medication for three months. Series 1 shows the average hours slept a night for each participant prior to medication. Series 2 shows the average hours slept a night for each participant after three months of use. The average hours slept a night was obtained by recording sleep every night for one month, and then finding the average. Patients one through five took a placebo pil, not the actual Moonlight medication. Note: The y-axis is measured in hours slept a night, while the x-axis lists each anonymous patient numerically.Q.The overall trend when comparing sleep before and after mediciation use is __________.a)There was not a difference in the average hours slept a night by a patient before the medication use and after the medication use.b)Patients who slept the most prior to the medication saw the least increase in hours slept when on the medication.c)Patients who slept the most prior to the medication saw the greatest increase in hours slept when on the medication.d)Patients who slept the least prior to the medication saw the greatest increase in hours slept when on the medication.e)All patients experienced an increase in hours slept when on the medication, except those taking the placebo.Correct answer is option 'D'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about A new drug is in its clinical trial phase. The graph below shows the data for participants sleep patterns before medication use and then after using the medication for three months. Series 1 shows the average hours slept a night for each participant prior to medication. Series 2 shows the average hours slept a night for each participant after three months of use. The average hours slept a night was obtained by recording sleep every night for one month, and then finding the average. Patients one through five took a placebo pil, not the actual Moonlight medication. Note: The y-axis is measured in hours slept a night, while the x-axis lists each anonymous patient numerically.Q.The overall trend when comparing sleep before and after mediciation use is __________.a)There was not a difference in the average hours slept a night by a patient before the medication use and after the medication use.b)Patients who slept the most prior to the medication saw the least increase in hours slept when on the medication.c)Patients who slept the most prior to the medication saw the greatest increase in hours slept when on the medication.d)Patients who slept the least prior to the medication saw the greatest increase in hours slept when on the medication.e)All patients experienced an increase in hours slept when on the medication, except those taking the placebo.Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A new drug is in its clinical trial phase. The graph below shows the data for participants sleep patterns before medication use and then after using the medication for three months. Series 1 shows the average hours slept a night for each participant prior to medication. Series 2 shows the average hours slept a night for each participant after three months of use. The average hours slept a night was obtained by recording sleep every night for one month, and then finding the average. Patients one through five took a placebo pil, not the actual Moonlight medication. Note: The y-axis is measured in hours slept a night, while the x-axis lists each anonymous patient numerically.Q.The overall trend when comparing sleep before and after mediciation use is __________.a)There was not a difference in the average hours slept a night by a patient before the medication use and after the medication use.b)Patients who slept the most prior to the medication saw the least increase in hours slept when on the medication.c)Patients who slept the most prior to the medication saw the greatest increase in hours slept when on the medication.d)Patients who slept the least prior to the medication saw the greatest increase in hours slept when on the medication.e)All patients experienced an increase in hours slept when on the medication, except those taking the placebo.Correct answer is option 'D'. Can you explain this answer?.
A new drug is in its clinical trial phase. The graph below shows the data for participants sleep patterns before medication use and then after using the medication for three months. Series 1 shows the average hours slept a night for each participant prior to medication. Series 2 shows the average hours slept a night for each participant after three months of use. The average hours slept a night was obtained by recording sleep every night for one month, and then finding the average. Patients one through five took a placebo pil, not the actual Moonlight medication. Note: The y-axis is measured in hours slept a night, while the x-axis lists each anonymous patient numerically.Q.The overall trend when comparing sleep before and after mediciation use is __________.a)There was not a difference in the average hours slept a night by a patient before the medication use and after the medication use.b)Patients who slept the most prior to the medication saw the least increase in hours slept when on the medication.c)Patients who slept the most prior to the medication saw the greatest increase in hours slept when on the medication.d)Patients who slept the least prior to the medication saw the greatest increase in hours slept when on the medication.e)All patients experienced an increase in hours slept when on the medication, except those taking the placebo.Correct answer is option 'D'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about A new drug is in its clinical trial phase. The graph below shows the data for participants sleep patterns before medication use and then after using the medication for three months. Series 1 shows the average hours slept a night for each participant prior to medication. Series 2 shows the average hours slept a night for each participant after three months of use. The average hours slept a night was obtained by recording sleep every night for one month, and then finding the average. Patients one through five took a placebo pil, not the actual Moonlight medication. Note: The y-axis is measured in hours slept a night, while the x-axis lists each anonymous patient numerically.Q.The overall trend when comparing sleep before and after mediciation use is __________.a)There was not a difference in the average hours slept a night by a patient before the medication use and after the medication use.b)Patients who slept the most prior to the medication saw the least increase in hours slept when on the medication.c)Patients who slept the most prior to the medication saw the greatest increase in hours slept when on the medication.d)Patients who slept the least prior to the medication saw the greatest increase in hours slept when on the medication.e)All patients experienced an increase in hours slept when on the medication, except those taking the placebo.Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for A new drug is in its clinical trial phase. The graph below shows the data for participants sleep patterns before medication use and then after using the medication for three months. Series 1 shows the average hours slept a night for each participant prior to medication. Series 2 shows the average hours slept a night for each participant after three months of use. The average hours slept a night was obtained by recording sleep every night for one month, and then finding the average. Patients one through five took a placebo pil, not the actual Moonlight medication. Note: The y-axis is measured in hours slept a night, while the x-axis lists each anonymous patient numerically.Q.The overall trend when comparing sleep before and after mediciation use is __________.a)There was not a difference in the average hours slept a night by a patient before the medication use and after the medication use.b)Patients who slept the most prior to the medication saw the least increase in hours slept when on the medication.c)Patients who slept the most prior to the medication saw the greatest increase in hours slept when on the medication.d)Patients who slept the least prior to the medication saw the greatest increase in hours slept when on the medication.e)All patients experienced an increase in hours slept when on the medication, except those taking the placebo.Correct answer is option 'D'. Can you explain this answer?.
Solutions for A new drug is in its clinical trial phase. The graph below shows the data for participants sleep patterns before medication use and then after using the medication for three months. Series 1 shows the average hours slept a night for each participant prior to medication. Series 2 shows the average hours slept a night for each participant after three months of use. The average hours slept a night was obtained by recording sleep every night for one month, and then finding the average. Patients one through five took a placebo pil, not the actual Moonlight medication. Note: The y-axis is measured in hours slept a night, while the x-axis lists each anonymous patient numerically.Q.The overall trend when comparing sleep before and after mediciation use is __________.a)There was not a difference in the average hours slept a night by a patient before the medication use and after the medication use.b)Patients who slept the most prior to the medication saw the least increase in hours slept when on the medication.c)Patients who slept the most prior to the medication saw the greatest increase in hours slept when on the medication.d)Patients who slept the least prior to the medication saw the greatest increase in hours slept when on the medication.e)All patients experienced an increase in hours slept when on the medication, except those taking the placebo.Correct answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for ACT.
Download more important topics, notes, lectures and mock test series for ACT Exam by signing up for free.
Here you can find the meaning of A new drug is in its clinical trial phase. The graph below shows the data for participants sleep patterns before medication use and then after using the medication for three months. Series 1 shows the average hours slept a night for each participant prior to medication. Series 2 shows the average hours slept a night for each participant after three months of use. The average hours slept a night was obtained by recording sleep every night for one month, and then finding the average. Patients one through five took a placebo pil, not the actual Moonlight medication. Note: The y-axis is measured in hours slept a night, while the x-axis lists each anonymous patient numerically.Q.The overall trend when comparing sleep before and after mediciation use is __________.a)There was not a difference in the average hours slept a night by a patient before the medication use and after the medication use.b)Patients who slept the most prior to the medication saw the least increase in hours slept when on the medication.c)Patients who slept the most prior to the medication saw the greatest increase in hours slept when on the medication.d)Patients who slept the least prior to the medication saw the greatest increase in hours slept when on the medication.e)All patients experienced an increase in hours slept when on the medication, except those taking the placebo.Correct answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
A new drug is in its clinical trial phase. The graph below shows the data for participants sleep patterns before medication use and then after using the medication for three months. Series 1 shows the average hours slept a night for each participant prior to medication. Series 2 shows the average hours slept a night for each participant after three months of use. The average hours slept a night was obtained by recording sleep every night for one month, and then finding the average. Patients one through five took a placebo pil, not the actual Moonlight medication. Note: The y-axis is measured in hours slept a night, while the x-axis lists each anonymous patient numerically.Q.The overall trend when comparing sleep before and after mediciation use is __________.a)There was not a difference in the average hours slept a night by a patient before the medication use and after the medication use.b)Patients who slept the most prior to the medication saw the least increase in hours slept when on the medication.c)Patients who slept the most prior to the medication saw the greatest increase in hours slept when on the medication.d)Patients who slept the least prior to the medication saw the greatest increase in hours slept when on the medication.e)All patients experienced an increase in hours slept when on the medication, except those taking the placebo.Correct answer is option 'D'. Can you explain this answer?, a detailed solution for A new drug is in its clinical trial phase. The graph below shows the data for participants sleep patterns before medication use and then after using the medication for three months. Series 1 shows the average hours slept a night for each participant prior to medication. Series 2 shows the average hours slept a night for each participant after three months of use. The average hours slept a night was obtained by recording sleep every night for one month, and then finding the average. Patients one through five took a placebo pil, not the actual Moonlight medication. Note: The y-axis is measured in hours slept a night, while the x-axis lists each anonymous patient numerically.Q.The overall trend when comparing sleep before and after mediciation use is __________.a)There was not a difference in the average hours slept a night by a patient before the medication use and after the medication use.b)Patients who slept the most prior to the medication saw the least increase in hours slept when on the medication.c)Patients who slept the most prior to the medication saw the greatest increase in hours slept when on the medication.d)Patients who slept the least prior to the medication saw the greatest increase in hours slept when on the medication.e)All patients experienced an increase in hours slept when on the medication, except those taking the placebo.Correct answer is option 'D'. Can you explain this answer? has been provided alongside types of A new drug is in its clinical trial phase. The graph below shows the data for participants sleep patterns before medication use and then after using the medication for three months. Series 1 shows the average hours slept a night for each participant prior to medication. Series 2 shows the average hours slept a night for each participant after three months of use. The average hours slept a night was obtained by recording sleep every night for one month, and then finding the average. Patients one through five took a placebo pil, not the actual Moonlight medication. Note: The y-axis is measured in hours slept a night, while the x-axis lists each anonymous patient numerically.Q.The overall trend when comparing sleep before and after mediciation use is __________.a)There was not a difference in the average hours slept a night by a patient before the medication use and after the medication use.b)Patients who slept the most prior to the medication saw the least increase in hours slept when on the medication.c)Patients who slept the most prior to the medication saw the greatest increase in hours slept when on the medication.d)Patients who slept the least prior to the medication saw the greatest increase in hours slept when on the medication.e)All patients experienced an increase in hours slept when on the medication, except those taking the placebo.Correct answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice A new drug is in its clinical trial phase. The graph below shows the data for participants sleep patterns before medication use and then after using the medication for three months. Series 1 shows the average hours slept a night for each participant prior to medication. Series 2 shows the average hours slept a night for each participant after three months of use. The average hours slept a night was obtained by recording sleep every night for one month, and then finding the average. Patients one through five took a placebo pil, not the actual Moonlight medication. Note: The y-axis is measured in hours slept a night, while the x-axis lists each anonymous patient numerically.Q.The overall trend when comparing sleep before and after mediciation use is __________.a)There was not a difference in the average hours slept a night by a patient before the medication use and after the medication use.b)Patients who slept the most prior to the medication saw the least increase in hours slept when on the medication.c)Patients who slept the most prior to the medication saw the greatest increase in hours slept when on the medication.d)Patients who slept the least prior to the medication saw the greatest increase in hours slept when on the medication.e)All patients experienced an increase in hours slept when on the medication, except those taking the placebo.Correct answer is option 'D'. Can you explain this answer? tests, examples and also practice ACT tests.

|
Explore Courses for ACT exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























