SAT Exam > SAT Questions > Question based on the following passage.This ...
Start Learning for Free
Question based on the following passage.
This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.
As any good contractor will tell you, a sound
structure requires stable materials. But atoms,
the building blocks of everything we know and
love—bunnies, brownies, and best friends—
(5) don't appear to be models of stability. Why are
some atoms, like sodium, so hyperactive while
others, like helium, are so aloof? Why do the
electrons that inhabit atoms jump around so
strangely, from one bizarrely shaped orbital to
(10) another? And why do protons, the bits that
give atoms their heft and personality, stick
together at all?
We are told that every atom has a tiny
nucleus containing positively charged protons
(15) and uncharged neutrons, swarmed by a cloud
of speedy electrons. We are also told that like
charges, such as protons, repel each other with
a force that shoots up to infinity as they get
closer. Even worse, you can't get much closer
(20) than two protons in the nucleus of an atom. So
what's keeping atomic nuclei from flying apart?
Obviously, some other force must be at work
inside the atom, something that we can't detect at
our human scale. Physicists call this the
(25) “strong nuclear force.” But where does it
come from?
In order for this force to account for the
binding of protons in the nucleus, it must have
certain interesting features. First, it can't have any
(30) sizeable effect beyond the radius of the atom itself,
or it would play havoc with the nuclei of adjacent
atoms, destroying matter as we know it. Second,
it must perfectly balance the repulsive force of
electricity at an “equilibrium point” of about
(35) 0.7 x 10-15 meters, the average distance between
bound protons, in order to create a stable nucleus.
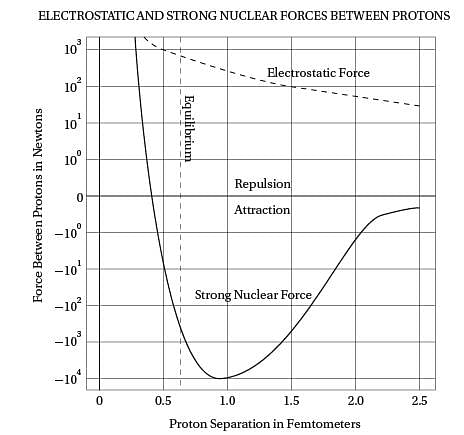
Third, it must repel at even shorter distances, or
else neutrons (which don't have any electrostatic
repulsion to balance the strong nuclear force)
(40) would collapse into each other. The graph
shows the behavior of such a force relative
to the repulsive electrostatic force.
In 1935, Japanese physicist Hideki Yukawa
proposed that the nuclear force was conveyed by
(45) a then-undiscovered heavy subatomic particle
he called the pi meson (or “pion”), which (unlike
the photon, which conveys the electrostatic
force) decays very quickly and therefore conveys a
powerful force only over a very short distance.
(50) Professor Yukawa's theory, however, was
dealt a mortal blow by a series of experiments
conducted at Los Alamos National Laboratory
in the early 1990s that demonstrated that pions
carry force only over distances greater than the
(55) distance between bound protons. The pion was a
plumber's wrench trying to do a tweezer's job.
Current atomic theory suggests that the
strong nuclear force is most likely conveyed by
massless particles called “gluons” according
(60) to the theory of quantum chromodynamics, or
QCD for short. According to QCD, protons and
neutrons are composed of smaller particles
called quarks, which are held together by the
aptly named gluons. This quark-binding force has
(65) a “residue” that extends beyond the protons and
neutrons themselves to provide just enough force
to bind the protons and neutrons together.
If you're hoping that QCD ties up atomic
behavior with a tidy little bow, you may be just
(70) a bit disappointed. As a quantum theory, it
conceives of space and time as tiny chunks that
occasionally misbehave, rather than smooth
predictable quantities, and its mathematical
formulas are perhaps as hard to penetrate as the
(75) nucleus itself.
This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.
As any good contractor will tell you, a sound
structure requires stable materials. But atoms,
the building blocks of everything we know and
love—bunnies, brownies, and best friends—
(5) don't appear to be models of stability. Why are
some atoms, like sodium, so hyperactive while
others, like helium, are so aloof? Why do the
electrons that inhabit atoms jump around so
strangely, from one bizarrely shaped orbital to
(10) another? And why do protons, the bits that
give atoms their heft and personality, stick
together at all?
We are told that every atom has a tiny
nucleus containing positively charged protons
(15) and uncharged neutrons, swarmed by a cloud
of speedy electrons. We are also told that like
charges, such as protons, repel each other with
a force that shoots up to infinity as they get
closer. Even worse, you can't get much closer
(20) than two protons in the nucleus of an atom. So
what's keeping atomic nuclei from flying apart?
Obviously, some other force must be at work
inside the atom, something that we can't detect at
our human scale. Physicists call this the
(25) “strong nuclear force.” But where does it
come from?
In order for this force to account for the
binding of protons in the nucleus, it must have
certain interesting features. First, it can't have any
(30) sizeable effect beyond the radius of the atom itself,
or it would play havoc with the nuclei of adjacent
atoms, destroying matter as we know it. Second,
it must perfectly balance the repulsive force of
electricity at an “equilibrium point” of about
(35) 0.7 x 10-15 meters, the average distance between
bound protons, in order to create a stable nucleus.
Third, it must repel at even shorter distances, or
else neutrons (which don't have any electrostatic
repulsion to balance the strong nuclear force)
(40) would collapse into each other. The graph
shows the behavior of such a force relative
to the repulsive electrostatic force.
In 1935, Japanese physicist Hideki Yukawa
proposed that the nuclear force was conveyed by
(45) a then-undiscovered heavy subatomic particle
he called the pi meson (or “pion”), which (unlike
the photon, which conveys the electrostatic
force) decays very quickly and therefore conveys a
powerful force only over a very short distance.
(50) Professor Yukawa's theory, however, was
dealt a mortal blow by a series of experiments
conducted at Los Alamos National Laboratory
in the early 1990s that demonstrated that pions
carry force only over distances greater than the
(55) distance between bound protons. The pion was a
plumber's wrench trying to do a tweezer's job.
Current atomic theory suggests that the
strong nuclear force is most likely conveyed by
massless particles called “gluons” according
(60) to the theory of quantum chromodynamics, or
QCD for short. According to QCD, protons and
neutrons are composed of smaller particles
called quarks, which are held together by the
aptly named gluons. This quark-binding force has
(65) a “residue” that extends beyond the protons and
neutrons themselves to provide just enough force
to bind the protons and neutrons together.
If you're hoping that QCD ties up atomic
behavior with a tidy little bow, you may be just
(70) a bit disappointed. As a quantum theory, it
conceives of space and time as tiny chunks that
occasionally misbehave, rather than smooth
predictable quantities, and its mathematical
formulas are perhaps as hard to penetrate as the
(75) nucleus itself.

Q. Which of the following best describes the structure of the passage as a whole?
- a)a series of intuitive illustrations of a complex physical theory
- b)a description of a technical puzzle and the attempts to solve it
- c)an account of an experimental finding and its surprising implications
- d)a historical over view of a heated scientific controversy
Correct answer is option 'B'. Can you explain this answer?
Most Upvoted Answer
Question based on the following passage.This passage is from S. K. Muk...
The first paragraph of this passage introduces the scientific conundrum of how protons adhere in atomic nuclei. The second paragraph analyzes this strange situation.
The third paragraph describes a force, the strong nuclear force, that could solve the conundrum. The fourth paragraph describes a particular theory, now refuted, about what might convey this strong nuclear force. The fifth and sixth paragraphs indicate that the problem has yet to be satisfactorily resolved. Thus, the passage as a whole is a description of a technical puzzle and the attempts to solve it.
The third paragraph describes a force, the strong nuclear force, that could solve the conundrum. The fourth paragraph describes a particular theory, now refuted, about what might convey this strong nuclear force. The fifth and sixth paragraphs indicate that the problem has yet to be satisfactorily resolved. Thus, the passage as a whole is a description of a technical puzzle and the attempts to solve it.

|
Explore Courses for SAT exam
|

|
Question based on the following passage.This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.As any good contractor will tell you, a soundstructure requires stable materials. But atoms,the building blocks of everything we know andlove—bunnies, brownies, and best friends—(5) dont appear to be models of stability. Why aresome atoms, like sodium, so hyperactive whileothers, like helium, are so aloof? Why do theelectrons that inhabit atoms jump around sostrangely, from one bizarrely shaped orbital to(10) another? And why do protons, the bits thatgive atoms their heft and personality, sticktogether at all?We are told that every atom has a tinynucleus containing positively charged protons(15) and uncharged neutrons, swarmed by a cloudof speedy electrons. We are also told that likecharges, such as protons, repel each other witha force that shoots up to infinity as they getcloser. Even worse, you cant get much closer(20) than two protons in the nucleus of an atom. Sowhats keeping atomic nuclei from flying apart?Obviously, some other force must be at workinside the atom, something that we cant detect atour human scale. Physicists call this the(25) “strong nuclear force.” But where does itcome from?In order for this force to account for thebinding of protons in the nucleus, it must havecertain interesting features. First, it cant have any(30) sizeable effect beyond the radius of the atom itself,or it would play havoc with the nuclei of adjacentatoms, destroying matter as we know it. Second,it must perfectly balance the repulsive force ofelectricity at an “equilibrium point” of about(35) 0.7 x 10-15 meters, the average distance betweenbound protons, in order to create a stable nucleus.Third, it must repel at even shorter distances, orelse neutrons (which dont have any electrostaticrepulsion to balance the strong nuclear force)(40) would collapse into each other. The graphshows the behavior of such a force relativeto the repulsive electrostatic force.In 1935, Japanese physicist Hideki Yukawaproposed that the nuclear force was conveyed by(45) a then-undiscovered heavy subatomic particlehe called the pi meson (or “pion”), which (unlikethe photon, which conveys the electrostaticforce) decays very quickly and therefore conveys apowerful force only over a very short distance.(50) Professor Yukawas theory, however, wasdealt a mortal blow by a series of experimentsconducted at Los Alamos National Laboratoryin the early 1990s that demonstrated that pionscarry force only over distances greater than the(55) distance between bound protons. The pion was aplumbers wrench trying to do a tweezers job.Current atomic theory suggests that thestrong nuclear force is most likely conveyed bymassless particles called “gluons” according(60) to the theory of quantum chromodynamics, orQCD for short. According to QCD, protons andneutrons are composed of smaller particlescalled quarks, which are held together by theaptly named gluons. This quark-binding force has(65) a “residue” that extends beyond the protons andneutrons themselves to provide just enough forceto bind the protons and neutrons together.If youre hoping that QCD ties up atomicbehavior with a tidy little bow, you may be just(70) a bit disappointed. As a quantum theory, itconceives of space and time as tiny chunks thatoccasionally misbehave, rather than smoothpredictable quantities, and its mathematicalformulas are perhaps as hard to penetrate as the(75) nucleus itself.Q.Which of the following best describes the structure of the passage as a whole?a)a series of intuitive illustrations of a complex physical theoryb)a description of a technical puzzle and the attempts to solve itc)an account of an experimental finding and its surprising implicationsd)a historical over view of a heated scientific controversyCorrect answer is option 'B'. Can you explain this answer?
Question Description
Question based on the following passage.This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.As any good contractor will tell you, a soundstructure requires stable materials. But atoms,the building blocks of everything we know andlove—bunnies, brownies, and best friends—(5) dont appear to be models of stability. Why aresome atoms, like sodium, so hyperactive whileothers, like helium, are so aloof? Why do theelectrons that inhabit atoms jump around sostrangely, from one bizarrely shaped orbital to(10) another? And why do protons, the bits thatgive atoms their heft and personality, sticktogether at all?We are told that every atom has a tinynucleus containing positively charged protons(15) and uncharged neutrons, swarmed by a cloudof speedy electrons. We are also told that likecharges, such as protons, repel each other witha force that shoots up to infinity as they getcloser. Even worse, you cant get much closer(20) than two protons in the nucleus of an atom. Sowhats keeping atomic nuclei from flying apart?Obviously, some other force must be at workinside the atom, something that we cant detect atour human scale. Physicists call this the(25) “strong nuclear force.” But where does itcome from?In order for this force to account for thebinding of protons in the nucleus, it must havecertain interesting features. First, it cant have any(30) sizeable effect beyond the radius of the atom itself,or it would play havoc with the nuclei of adjacentatoms, destroying matter as we know it. Second,it must perfectly balance the repulsive force ofelectricity at an “equilibrium point” of about(35) 0.7 x 10-15 meters, the average distance betweenbound protons, in order to create a stable nucleus.Third, it must repel at even shorter distances, orelse neutrons (which dont have any electrostaticrepulsion to balance the strong nuclear force)(40) would collapse into each other. The graphshows the behavior of such a force relativeto the repulsive electrostatic force.In 1935, Japanese physicist Hideki Yukawaproposed that the nuclear force was conveyed by(45) a then-undiscovered heavy subatomic particlehe called the pi meson (or “pion”), which (unlikethe photon, which conveys the electrostaticforce) decays very quickly and therefore conveys apowerful force only over a very short distance.(50) Professor Yukawas theory, however, wasdealt a mortal blow by a series of experimentsconducted at Los Alamos National Laboratoryin the early 1990s that demonstrated that pionscarry force only over distances greater than the(55) distance between bound protons. The pion was aplumbers wrench trying to do a tweezers job.Current atomic theory suggests that thestrong nuclear force is most likely conveyed bymassless particles called “gluons” according(60) to the theory of quantum chromodynamics, orQCD for short. According to QCD, protons andneutrons are composed of smaller particlescalled quarks, which are held together by theaptly named gluons. This quark-binding force has(65) a “residue” that extends beyond the protons andneutrons themselves to provide just enough forceto bind the protons and neutrons together.If youre hoping that QCD ties up atomicbehavior with a tidy little bow, you may be just(70) a bit disappointed. As a quantum theory, itconceives of space and time as tiny chunks thatoccasionally misbehave, rather than smoothpredictable quantities, and its mathematicalformulas are perhaps as hard to penetrate as the(75) nucleus itself.Q.Which of the following best describes the structure of the passage as a whole?a)a series of intuitive illustrations of a complex physical theoryb)a description of a technical puzzle and the attempts to solve itc)an account of an experimental finding and its surprising implicationsd)a historical over view of a heated scientific controversyCorrect answer is option 'B'. Can you explain this answer? for SAT 2025 is part of SAT preparation. The Question and answers have been prepared according to the SAT exam syllabus. Information about Question based on the following passage.This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.As any good contractor will tell you, a soundstructure requires stable materials. But atoms,the building blocks of everything we know andlove—bunnies, brownies, and best friends—(5) dont appear to be models of stability. Why aresome atoms, like sodium, so hyperactive whileothers, like helium, are so aloof? Why do theelectrons that inhabit atoms jump around sostrangely, from one bizarrely shaped orbital to(10) another? And why do protons, the bits thatgive atoms their heft and personality, sticktogether at all?We are told that every atom has a tinynucleus containing positively charged protons(15) and uncharged neutrons, swarmed by a cloudof speedy electrons. We are also told that likecharges, such as protons, repel each other witha force that shoots up to infinity as they getcloser. Even worse, you cant get much closer(20) than two protons in the nucleus of an atom. Sowhats keeping atomic nuclei from flying apart?Obviously, some other force must be at workinside the atom, something that we cant detect atour human scale. Physicists call this the(25) “strong nuclear force.” But where does itcome from?In order for this force to account for thebinding of protons in the nucleus, it must havecertain interesting features. First, it cant have any(30) sizeable effect beyond the radius of the atom itself,or it would play havoc with the nuclei of adjacentatoms, destroying matter as we know it. Second,it must perfectly balance the repulsive force ofelectricity at an “equilibrium point” of about(35) 0.7 x 10-15 meters, the average distance betweenbound protons, in order to create a stable nucleus.Third, it must repel at even shorter distances, orelse neutrons (which dont have any electrostaticrepulsion to balance the strong nuclear force)(40) would collapse into each other. The graphshows the behavior of such a force relativeto the repulsive electrostatic force.In 1935, Japanese physicist Hideki Yukawaproposed that the nuclear force was conveyed by(45) a then-undiscovered heavy subatomic particlehe called the pi meson (or “pion”), which (unlikethe photon, which conveys the electrostaticforce) decays very quickly and therefore conveys apowerful force only over a very short distance.(50) Professor Yukawas theory, however, wasdealt a mortal blow by a series of experimentsconducted at Los Alamos National Laboratoryin the early 1990s that demonstrated that pionscarry force only over distances greater than the(55) distance between bound protons. The pion was aplumbers wrench trying to do a tweezers job.Current atomic theory suggests that thestrong nuclear force is most likely conveyed bymassless particles called “gluons” according(60) to the theory of quantum chromodynamics, orQCD for short. According to QCD, protons andneutrons are composed of smaller particlescalled quarks, which are held together by theaptly named gluons. This quark-binding force has(65) a “residue” that extends beyond the protons andneutrons themselves to provide just enough forceto bind the protons and neutrons together.If youre hoping that QCD ties up atomicbehavior with a tidy little bow, you may be just(70) a bit disappointed. As a quantum theory, itconceives of space and time as tiny chunks thatoccasionally misbehave, rather than smoothpredictable quantities, and its mathematicalformulas are perhaps as hard to penetrate as the(75) nucleus itself.Q.Which of the following best describes the structure of the passage as a whole?a)a series of intuitive illustrations of a complex physical theoryb)a description of a technical puzzle and the attempts to solve itc)an account of an experimental finding and its surprising implicationsd)a historical over view of a heated scientific controversyCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for SAT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Question based on the following passage.This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.As any good contractor will tell you, a soundstructure requires stable materials. But atoms,the building blocks of everything we know andlove—bunnies, brownies, and best friends—(5) dont appear to be models of stability. Why aresome atoms, like sodium, so hyperactive whileothers, like helium, are so aloof? Why do theelectrons that inhabit atoms jump around sostrangely, from one bizarrely shaped orbital to(10) another? And why do protons, the bits thatgive atoms their heft and personality, sticktogether at all?We are told that every atom has a tinynucleus containing positively charged protons(15) and uncharged neutrons, swarmed by a cloudof speedy electrons. We are also told that likecharges, such as protons, repel each other witha force that shoots up to infinity as they getcloser. Even worse, you cant get much closer(20) than two protons in the nucleus of an atom. Sowhats keeping atomic nuclei from flying apart?Obviously, some other force must be at workinside the atom, something that we cant detect atour human scale. Physicists call this the(25) “strong nuclear force.” But where does itcome from?In order for this force to account for thebinding of protons in the nucleus, it must havecertain interesting features. First, it cant have any(30) sizeable effect beyond the radius of the atom itself,or it would play havoc with the nuclei of adjacentatoms, destroying matter as we know it. Second,it must perfectly balance the repulsive force ofelectricity at an “equilibrium point” of about(35) 0.7 x 10-15 meters, the average distance betweenbound protons, in order to create a stable nucleus.Third, it must repel at even shorter distances, orelse neutrons (which dont have any electrostaticrepulsion to balance the strong nuclear force)(40) would collapse into each other. The graphshows the behavior of such a force relativeto the repulsive electrostatic force.In 1935, Japanese physicist Hideki Yukawaproposed that the nuclear force was conveyed by(45) a then-undiscovered heavy subatomic particlehe called the pi meson (or “pion”), which (unlikethe photon, which conveys the electrostaticforce) decays very quickly and therefore conveys apowerful force only over a very short distance.(50) Professor Yukawas theory, however, wasdealt a mortal blow by a series of experimentsconducted at Los Alamos National Laboratoryin the early 1990s that demonstrated that pionscarry force only over distances greater than the(55) distance between bound protons. The pion was aplumbers wrench trying to do a tweezers job.Current atomic theory suggests that thestrong nuclear force is most likely conveyed bymassless particles called “gluons” according(60) to the theory of quantum chromodynamics, orQCD for short. According to QCD, protons andneutrons are composed of smaller particlescalled quarks, which are held together by theaptly named gluons. This quark-binding force has(65) a “residue” that extends beyond the protons andneutrons themselves to provide just enough forceto bind the protons and neutrons together.If youre hoping that QCD ties up atomicbehavior with a tidy little bow, you may be just(70) a bit disappointed. As a quantum theory, itconceives of space and time as tiny chunks thatoccasionally misbehave, rather than smoothpredictable quantities, and its mathematicalformulas are perhaps as hard to penetrate as the(75) nucleus itself.Q.Which of the following best describes the structure of the passage as a whole?a)a series of intuitive illustrations of a complex physical theoryb)a description of a technical puzzle and the attempts to solve itc)an account of an experimental finding and its surprising implicationsd)a historical over view of a heated scientific controversyCorrect answer is option 'B'. Can you explain this answer?.
Question based on the following passage.This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.As any good contractor will tell you, a soundstructure requires stable materials. But atoms,the building blocks of everything we know andlove—bunnies, brownies, and best friends—(5) dont appear to be models of stability. Why aresome atoms, like sodium, so hyperactive whileothers, like helium, are so aloof? Why do theelectrons that inhabit atoms jump around sostrangely, from one bizarrely shaped orbital to(10) another? And why do protons, the bits thatgive atoms their heft and personality, sticktogether at all?We are told that every atom has a tinynucleus containing positively charged protons(15) and uncharged neutrons, swarmed by a cloudof speedy electrons. We are also told that likecharges, such as protons, repel each other witha force that shoots up to infinity as they getcloser. Even worse, you cant get much closer(20) than two protons in the nucleus of an atom. Sowhats keeping atomic nuclei from flying apart?Obviously, some other force must be at workinside the atom, something that we cant detect atour human scale. Physicists call this the(25) “strong nuclear force.” But where does itcome from?In order for this force to account for thebinding of protons in the nucleus, it must havecertain interesting features. First, it cant have any(30) sizeable effect beyond the radius of the atom itself,or it would play havoc with the nuclei of adjacentatoms, destroying matter as we know it. Second,it must perfectly balance the repulsive force ofelectricity at an “equilibrium point” of about(35) 0.7 x 10-15 meters, the average distance betweenbound protons, in order to create a stable nucleus.Third, it must repel at even shorter distances, orelse neutrons (which dont have any electrostaticrepulsion to balance the strong nuclear force)(40) would collapse into each other. The graphshows the behavior of such a force relativeto the repulsive electrostatic force.In 1935, Japanese physicist Hideki Yukawaproposed that the nuclear force was conveyed by(45) a then-undiscovered heavy subatomic particlehe called the pi meson (or “pion”), which (unlikethe photon, which conveys the electrostaticforce) decays very quickly and therefore conveys apowerful force only over a very short distance.(50) Professor Yukawas theory, however, wasdealt a mortal blow by a series of experimentsconducted at Los Alamos National Laboratoryin the early 1990s that demonstrated that pionscarry force only over distances greater than the(55) distance between bound protons. The pion was aplumbers wrench trying to do a tweezers job.Current atomic theory suggests that thestrong nuclear force is most likely conveyed bymassless particles called “gluons” according(60) to the theory of quantum chromodynamics, orQCD for short. According to QCD, protons andneutrons are composed of smaller particlescalled quarks, which are held together by theaptly named gluons. This quark-binding force has(65) a “residue” that extends beyond the protons andneutrons themselves to provide just enough forceto bind the protons and neutrons together.If youre hoping that QCD ties up atomicbehavior with a tidy little bow, you may be just(70) a bit disappointed. As a quantum theory, itconceives of space and time as tiny chunks thatoccasionally misbehave, rather than smoothpredictable quantities, and its mathematicalformulas are perhaps as hard to penetrate as the(75) nucleus itself.Q.Which of the following best describes the structure of the passage as a whole?a)a series of intuitive illustrations of a complex physical theoryb)a description of a technical puzzle and the attempts to solve itc)an account of an experimental finding and its surprising implicationsd)a historical over view of a heated scientific controversyCorrect answer is option 'B'. Can you explain this answer? for SAT 2025 is part of SAT preparation. The Question and answers have been prepared according to the SAT exam syllabus. Information about Question based on the following passage.This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.As any good contractor will tell you, a soundstructure requires stable materials. But atoms,the building blocks of everything we know andlove—bunnies, brownies, and best friends—(5) dont appear to be models of stability. Why aresome atoms, like sodium, so hyperactive whileothers, like helium, are so aloof? Why do theelectrons that inhabit atoms jump around sostrangely, from one bizarrely shaped orbital to(10) another? And why do protons, the bits thatgive atoms their heft and personality, sticktogether at all?We are told that every atom has a tinynucleus containing positively charged protons(15) and uncharged neutrons, swarmed by a cloudof speedy electrons. We are also told that likecharges, such as protons, repel each other witha force that shoots up to infinity as they getcloser. Even worse, you cant get much closer(20) than two protons in the nucleus of an atom. Sowhats keeping atomic nuclei from flying apart?Obviously, some other force must be at workinside the atom, something that we cant detect atour human scale. Physicists call this the(25) “strong nuclear force.” But where does itcome from?In order for this force to account for thebinding of protons in the nucleus, it must havecertain interesting features. First, it cant have any(30) sizeable effect beyond the radius of the atom itself,or it would play havoc with the nuclei of adjacentatoms, destroying matter as we know it. Second,it must perfectly balance the repulsive force ofelectricity at an “equilibrium point” of about(35) 0.7 x 10-15 meters, the average distance betweenbound protons, in order to create a stable nucleus.Third, it must repel at even shorter distances, orelse neutrons (which dont have any electrostaticrepulsion to balance the strong nuclear force)(40) would collapse into each other. The graphshows the behavior of such a force relativeto the repulsive electrostatic force.In 1935, Japanese physicist Hideki Yukawaproposed that the nuclear force was conveyed by(45) a then-undiscovered heavy subatomic particlehe called the pi meson (or “pion”), which (unlikethe photon, which conveys the electrostaticforce) decays very quickly and therefore conveys apowerful force only over a very short distance.(50) Professor Yukawas theory, however, wasdealt a mortal blow by a series of experimentsconducted at Los Alamos National Laboratoryin the early 1990s that demonstrated that pionscarry force only over distances greater than the(55) distance between bound protons. The pion was aplumbers wrench trying to do a tweezers job.Current atomic theory suggests that thestrong nuclear force is most likely conveyed bymassless particles called “gluons” according(60) to the theory of quantum chromodynamics, orQCD for short. According to QCD, protons andneutrons are composed of smaller particlescalled quarks, which are held together by theaptly named gluons. This quark-binding force has(65) a “residue” that extends beyond the protons andneutrons themselves to provide just enough forceto bind the protons and neutrons together.If youre hoping that QCD ties up atomicbehavior with a tidy little bow, you may be just(70) a bit disappointed. As a quantum theory, itconceives of space and time as tiny chunks thatoccasionally misbehave, rather than smoothpredictable quantities, and its mathematicalformulas are perhaps as hard to penetrate as the(75) nucleus itself.Q.Which of the following best describes the structure of the passage as a whole?a)a series of intuitive illustrations of a complex physical theoryb)a description of a technical puzzle and the attempts to solve itc)an account of an experimental finding and its surprising implicationsd)a historical over view of a heated scientific controversyCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for SAT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Question based on the following passage.This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.As any good contractor will tell you, a soundstructure requires stable materials. But atoms,the building blocks of everything we know andlove—bunnies, brownies, and best friends—(5) dont appear to be models of stability. Why aresome atoms, like sodium, so hyperactive whileothers, like helium, are so aloof? Why do theelectrons that inhabit atoms jump around sostrangely, from one bizarrely shaped orbital to(10) another? And why do protons, the bits thatgive atoms their heft and personality, sticktogether at all?We are told that every atom has a tinynucleus containing positively charged protons(15) and uncharged neutrons, swarmed by a cloudof speedy electrons. We are also told that likecharges, such as protons, repel each other witha force that shoots up to infinity as they getcloser. Even worse, you cant get much closer(20) than two protons in the nucleus of an atom. Sowhats keeping atomic nuclei from flying apart?Obviously, some other force must be at workinside the atom, something that we cant detect atour human scale. Physicists call this the(25) “strong nuclear force.” But where does itcome from?In order for this force to account for thebinding of protons in the nucleus, it must havecertain interesting features. First, it cant have any(30) sizeable effect beyond the radius of the atom itself,or it would play havoc with the nuclei of adjacentatoms, destroying matter as we know it. Second,it must perfectly balance the repulsive force ofelectricity at an “equilibrium point” of about(35) 0.7 x 10-15 meters, the average distance betweenbound protons, in order to create a stable nucleus.Third, it must repel at even shorter distances, orelse neutrons (which dont have any electrostaticrepulsion to balance the strong nuclear force)(40) would collapse into each other. The graphshows the behavior of such a force relativeto the repulsive electrostatic force.In 1935, Japanese physicist Hideki Yukawaproposed that the nuclear force was conveyed by(45) a then-undiscovered heavy subatomic particlehe called the pi meson (or “pion”), which (unlikethe photon, which conveys the electrostaticforce) decays very quickly and therefore conveys apowerful force only over a very short distance.(50) Professor Yukawas theory, however, wasdealt a mortal blow by a series of experimentsconducted at Los Alamos National Laboratoryin the early 1990s that demonstrated that pionscarry force only over distances greater than the(55) distance between bound protons. The pion was aplumbers wrench trying to do a tweezers job.Current atomic theory suggests that thestrong nuclear force is most likely conveyed bymassless particles called “gluons” according(60) to the theory of quantum chromodynamics, orQCD for short. According to QCD, protons andneutrons are composed of smaller particlescalled quarks, which are held together by theaptly named gluons. This quark-binding force has(65) a “residue” that extends beyond the protons andneutrons themselves to provide just enough forceto bind the protons and neutrons together.If youre hoping that QCD ties up atomicbehavior with a tidy little bow, you may be just(70) a bit disappointed. As a quantum theory, itconceives of space and time as tiny chunks thatoccasionally misbehave, rather than smoothpredictable quantities, and its mathematicalformulas are perhaps as hard to penetrate as the(75) nucleus itself.Q.Which of the following best describes the structure of the passage as a whole?a)a series of intuitive illustrations of a complex physical theoryb)a description of a technical puzzle and the attempts to solve itc)an account of an experimental finding and its surprising implicationsd)a historical over view of a heated scientific controversyCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Question based on the following passage.This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.As any good contractor will tell you, a soundstructure requires stable materials. But atoms,the building blocks of everything we know andlove—bunnies, brownies, and best friends—(5) dont appear to be models of stability. Why aresome atoms, like sodium, so hyperactive whileothers, like helium, are so aloof? Why do theelectrons that inhabit atoms jump around sostrangely, from one bizarrely shaped orbital to(10) another? And why do protons, the bits thatgive atoms their heft and personality, sticktogether at all?We are told that every atom has a tinynucleus containing positively charged protons(15) and uncharged neutrons, swarmed by a cloudof speedy electrons. We are also told that likecharges, such as protons, repel each other witha force that shoots up to infinity as they getcloser. Even worse, you cant get much closer(20) than two protons in the nucleus of an atom. Sowhats keeping atomic nuclei from flying apart?Obviously, some other force must be at workinside the atom, something that we cant detect atour human scale. Physicists call this the(25) “strong nuclear force.” But where does itcome from?In order for this force to account for thebinding of protons in the nucleus, it must havecertain interesting features. First, it cant have any(30) sizeable effect beyond the radius of the atom itself,or it would play havoc with the nuclei of adjacentatoms, destroying matter as we know it. Second,it must perfectly balance the repulsive force ofelectricity at an “equilibrium point” of about(35) 0.7 x 10-15 meters, the average distance betweenbound protons, in order to create a stable nucleus.Third, it must repel at even shorter distances, orelse neutrons (which dont have any electrostaticrepulsion to balance the strong nuclear force)(40) would collapse into each other. The graphshows the behavior of such a force relativeto the repulsive electrostatic force.In 1935, Japanese physicist Hideki Yukawaproposed that the nuclear force was conveyed by(45) a then-undiscovered heavy subatomic particlehe called the pi meson (or “pion”), which (unlikethe photon, which conveys the electrostaticforce) decays very quickly and therefore conveys apowerful force only over a very short distance.(50) Professor Yukawas theory, however, wasdealt a mortal blow by a series of experimentsconducted at Los Alamos National Laboratoryin the early 1990s that demonstrated that pionscarry force only over distances greater than the(55) distance between bound protons. The pion was aplumbers wrench trying to do a tweezers job.Current atomic theory suggests that thestrong nuclear force is most likely conveyed bymassless particles called “gluons” according(60) to the theory of quantum chromodynamics, orQCD for short. According to QCD, protons andneutrons are composed of smaller particlescalled quarks, which are held together by theaptly named gluons. This quark-binding force has(65) a “residue” that extends beyond the protons andneutrons themselves to provide just enough forceto bind the protons and neutrons together.If youre hoping that QCD ties up atomicbehavior with a tidy little bow, you may be just(70) a bit disappointed. As a quantum theory, itconceives of space and time as tiny chunks thatoccasionally misbehave, rather than smoothpredictable quantities, and its mathematicalformulas are perhaps as hard to penetrate as the(75) nucleus itself.Q.Which of the following best describes the structure of the passage as a whole?a)a series of intuitive illustrations of a complex physical theoryb)a description of a technical puzzle and the attempts to solve itc)an account of an experimental finding and its surprising implicationsd)a historical over view of a heated scientific controversyCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for SAT.
Download more important topics, notes, lectures and mock test series for SAT Exam by signing up for free.
Here you can find the meaning of Question based on the following passage.This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.As any good contractor will tell you, a soundstructure requires stable materials. But atoms,the building blocks of everything we know andlove—bunnies, brownies, and best friends—(5) dont appear to be models of stability. Why aresome atoms, like sodium, so hyperactive whileothers, like helium, are so aloof? Why do theelectrons that inhabit atoms jump around sostrangely, from one bizarrely shaped orbital to(10) another? And why do protons, the bits thatgive atoms their heft and personality, sticktogether at all?We are told that every atom has a tinynucleus containing positively charged protons(15) and uncharged neutrons, swarmed by a cloudof speedy electrons. We are also told that likecharges, such as protons, repel each other witha force that shoots up to infinity as they getcloser. Even worse, you cant get much closer(20) than two protons in the nucleus of an atom. Sowhats keeping atomic nuclei from flying apart?Obviously, some other force must be at workinside the atom, something that we cant detect atour human scale. Physicists call this the(25) “strong nuclear force.” But where does itcome from?In order for this force to account for thebinding of protons in the nucleus, it must havecertain interesting features. First, it cant have any(30) sizeable effect beyond the radius of the atom itself,or it would play havoc with the nuclei of adjacentatoms, destroying matter as we know it. Second,it must perfectly balance the repulsive force ofelectricity at an “equilibrium point” of about(35) 0.7 x 10-15 meters, the average distance betweenbound protons, in order to create a stable nucleus.Third, it must repel at even shorter distances, orelse neutrons (which dont have any electrostaticrepulsion to balance the strong nuclear force)(40) would collapse into each other. The graphshows the behavior of such a force relativeto the repulsive electrostatic force.In 1935, Japanese physicist Hideki Yukawaproposed that the nuclear force was conveyed by(45) a then-undiscovered heavy subatomic particlehe called the pi meson (or “pion”), which (unlikethe photon, which conveys the electrostaticforce) decays very quickly and therefore conveys apowerful force only over a very short distance.(50) Professor Yukawas theory, however, wasdealt a mortal blow by a series of experimentsconducted at Los Alamos National Laboratoryin the early 1990s that demonstrated that pionscarry force only over distances greater than the(55) distance between bound protons. The pion was aplumbers wrench trying to do a tweezers job.Current atomic theory suggests that thestrong nuclear force is most likely conveyed bymassless particles called “gluons” according(60) to the theory of quantum chromodynamics, orQCD for short. According to QCD, protons andneutrons are composed of smaller particlescalled quarks, which are held together by theaptly named gluons. This quark-binding force has(65) a “residue” that extends beyond the protons andneutrons themselves to provide just enough forceto bind the protons and neutrons together.If youre hoping that QCD ties up atomicbehavior with a tidy little bow, you may be just(70) a bit disappointed. As a quantum theory, itconceives of space and time as tiny chunks thatoccasionally misbehave, rather than smoothpredictable quantities, and its mathematicalformulas are perhaps as hard to penetrate as the(75) nucleus itself.Q.Which of the following best describes the structure of the passage as a whole?a)a series of intuitive illustrations of a complex physical theoryb)a description of a technical puzzle and the attempts to solve itc)an account of an experimental finding and its surprising implicationsd)a historical over view of a heated scientific controversyCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Question based on the following passage.This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.As any good contractor will tell you, a soundstructure requires stable materials. But atoms,the building blocks of everything we know andlove—bunnies, brownies, and best friends—(5) dont appear to be models of stability. Why aresome atoms, like sodium, so hyperactive whileothers, like helium, are so aloof? Why do theelectrons that inhabit atoms jump around sostrangely, from one bizarrely shaped orbital to(10) another? And why do protons, the bits thatgive atoms their heft and personality, sticktogether at all?We are told that every atom has a tinynucleus containing positively charged protons(15) and uncharged neutrons, swarmed by a cloudof speedy electrons. We are also told that likecharges, such as protons, repel each other witha force that shoots up to infinity as they getcloser. Even worse, you cant get much closer(20) than two protons in the nucleus of an atom. Sowhats keeping atomic nuclei from flying apart?Obviously, some other force must be at workinside the atom, something that we cant detect atour human scale. Physicists call this the(25) “strong nuclear force.” But where does itcome from?In order for this force to account for thebinding of protons in the nucleus, it must havecertain interesting features. First, it cant have any(30) sizeable effect beyond the radius of the atom itself,or it would play havoc with the nuclei of adjacentatoms, destroying matter as we know it. Second,it must perfectly balance the repulsive force ofelectricity at an “equilibrium point” of about(35) 0.7 x 10-15 meters, the average distance betweenbound protons, in order to create a stable nucleus.Third, it must repel at even shorter distances, orelse neutrons (which dont have any electrostaticrepulsion to balance the strong nuclear force)(40) would collapse into each other. The graphshows the behavior of such a force relativeto the repulsive electrostatic force.In 1935, Japanese physicist Hideki Yukawaproposed that the nuclear force was conveyed by(45) a then-undiscovered heavy subatomic particlehe called the pi meson (or “pion”), which (unlikethe photon, which conveys the electrostaticforce) decays very quickly and therefore conveys apowerful force only over a very short distance.(50) Professor Yukawas theory, however, wasdealt a mortal blow by a series of experimentsconducted at Los Alamos National Laboratoryin the early 1990s that demonstrated that pionscarry force only over distances greater than the(55) distance between bound protons. The pion was aplumbers wrench trying to do a tweezers job.Current atomic theory suggests that thestrong nuclear force is most likely conveyed bymassless particles called “gluons” according(60) to the theory of quantum chromodynamics, orQCD for short. According to QCD, protons andneutrons are composed of smaller particlescalled quarks, which are held together by theaptly named gluons. This quark-binding force has(65) a “residue” that extends beyond the protons andneutrons themselves to provide just enough forceto bind the protons and neutrons together.If youre hoping that QCD ties up atomicbehavior with a tidy little bow, you may be just(70) a bit disappointed. As a quantum theory, itconceives of space and time as tiny chunks thatoccasionally misbehave, rather than smoothpredictable quantities, and its mathematicalformulas are perhaps as hard to penetrate as the(75) nucleus itself.Q.Which of the following best describes the structure of the passage as a whole?a)a series of intuitive illustrations of a complex physical theoryb)a description of a technical puzzle and the attempts to solve itc)an account of an experimental finding and its surprising implicationsd)a historical over view of a heated scientific controversyCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Question based on the following passage.This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.As any good contractor will tell you, a soundstructure requires stable materials. But atoms,the building blocks of everything we know andlove—bunnies, brownies, and best friends—(5) dont appear to be models of stability. Why aresome atoms, like sodium, so hyperactive whileothers, like helium, are so aloof? Why do theelectrons that inhabit atoms jump around sostrangely, from one bizarrely shaped orbital to(10) another? And why do protons, the bits thatgive atoms their heft and personality, sticktogether at all?We are told that every atom has a tinynucleus containing positively charged protons(15) and uncharged neutrons, swarmed by a cloudof speedy electrons. We are also told that likecharges, such as protons, repel each other witha force that shoots up to infinity as they getcloser. Even worse, you cant get much closer(20) than two protons in the nucleus of an atom. Sowhats keeping atomic nuclei from flying apart?Obviously, some other force must be at workinside the atom, something that we cant detect atour human scale. Physicists call this the(25) “strong nuclear force.” But where does itcome from?In order for this force to account for thebinding of protons in the nucleus, it must havecertain interesting features. First, it cant have any(30) sizeable effect beyond the radius of the atom itself,or it would play havoc with the nuclei of adjacentatoms, destroying matter as we know it. Second,it must perfectly balance the repulsive force ofelectricity at an “equilibrium point” of about(35) 0.7 x 10-15 meters, the average distance betweenbound protons, in order to create a stable nucleus.Third, it must repel at even shorter distances, orelse neutrons (which dont have any electrostaticrepulsion to balance the strong nuclear force)(40) would collapse into each other. The graphshows the behavior of such a force relativeto the repulsive electrostatic force.In 1935, Japanese physicist Hideki Yukawaproposed that the nuclear force was conveyed by(45) a then-undiscovered heavy subatomic particlehe called the pi meson (or “pion”), which (unlikethe photon, which conveys the electrostaticforce) decays very quickly and therefore conveys apowerful force only over a very short distance.(50) Professor Yukawas theory, however, wasdealt a mortal blow by a series of experimentsconducted at Los Alamos National Laboratoryin the early 1990s that demonstrated that pionscarry force only over distances greater than the(55) distance between bound protons. The pion was aplumbers wrench trying to do a tweezers job.Current atomic theory suggests that thestrong nuclear force is most likely conveyed bymassless particles called “gluons” according(60) to the theory of quantum chromodynamics, orQCD for short. According to QCD, protons andneutrons are composed of smaller particlescalled quarks, which are held together by theaptly named gluons. This quark-binding force has(65) a “residue” that extends beyond the protons andneutrons themselves to provide just enough forceto bind the protons and neutrons together.If youre hoping that QCD ties up atomicbehavior with a tidy little bow, you may be just(70) a bit disappointed. As a quantum theory, itconceives of space and time as tiny chunks thatoccasionally misbehave, rather than smoothpredictable quantities, and its mathematicalformulas are perhaps as hard to penetrate as the(75) nucleus itself.Q.Which of the following best describes the structure of the passage as a whole?a)a series of intuitive illustrations of a complex physical theoryb)a description of a technical puzzle and the attempts to solve itc)an account of an experimental finding and its surprising implicationsd)a historical over view of a heated scientific controversyCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Question based on the following passage.This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.As any good contractor will tell you, a soundstructure requires stable materials. But atoms,the building blocks of everything we know andlove—bunnies, brownies, and best friends—(5) dont appear to be models of stability. Why aresome atoms, like sodium, so hyperactive whileothers, like helium, are so aloof? Why do theelectrons that inhabit atoms jump around sostrangely, from one bizarrely shaped orbital to(10) another? And why do protons, the bits thatgive atoms their heft and personality, sticktogether at all?We are told that every atom has a tinynucleus containing positively charged protons(15) and uncharged neutrons, swarmed by a cloudof speedy electrons. We are also told that likecharges, such as protons, repel each other witha force that shoots up to infinity as they getcloser. Even worse, you cant get much closer(20) than two protons in the nucleus of an atom. Sowhats keeping atomic nuclei from flying apart?Obviously, some other force must be at workinside the atom, something that we cant detect atour human scale. Physicists call this the(25) “strong nuclear force.” But where does itcome from?In order for this force to account for thebinding of protons in the nucleus, it must havecertain interesting features. First, it cant have any(30) sizeable effect beyond the radius of the atom itself,or it would play havoc with the nuclei of adjacentatoms, destroying matter as we know it. Second,it must perfectly balance the repulsive force ofelectricity at an “equilibrium point” of about(35) 0.7 x 10-15 meters, the average distance betweenbound protons, in order to create a stable nucleus.Third, it must repel at even shorter distances, orelse neutrons (which dont have any electrostaticrepulsion to balance the strong nuclear force)(40) would collapse into each other. The graphshows the behavior of such a force relativeto the repulsive electrostatic force.In 1935, Japanese physicist Hideki Yukawaproposed that the nuclear force was conveyed by(45) a then-undiscovered heavy subatomic particlehe called the pi meson (or “pion”), which (unlikethe photon, which conveys the electrostaticforce) decays very quickly and therefore conveys apowerful force only over a very short distance.(50) Professor Yukawas theory, however, wasdealt a mortal blow by a series of experimentsconducted at Los Alamos National Laboratoryin the early 1990s that demonstrated that pionscarry force only over distances greater than the(55) distance between bound protons. The pion was aplumbers wrench trying to do a tweezers job.Current atomic theory suggests that thestrong nuclear force is most likely conveyed bymassless particles called “gluons” according(60) to the theory of quantum chromodynamics, orQCD for short. According to QCD, protons andneutrons are composed of smaller particlescalled quarks, which are held together by theaptly named gluons. This quark-binding force has(65) a “residue” that extends beyond the protons andneutrons themselves to provide just enough forceto bind the protons and neutrons together.If youre hoping that QCD ties up atomicbehavior with a tidy little bow, you may be just(70) a bit disappointed. As a quantum theory, itconceives of space and time as tiny chunks thatoccasionally misbehave, rather than smoothpredictable quantities, and its mathematicalformulas are perhaps as hard to penetrate as the(75) nucleus itself.Q.Which of the following best describes the structure of the passage as a whole?a)a series of intuitive illustrations of a complex physical theoryb)a description of a technical puzzle and the attempts to solve itc)an account of an experimental finding and its surprising implicationsd)a historical over view of a heated scientific controversyCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Question based on the following passage.This passage is from S. K. Mukherjee, “The Mysteries of the Strong Nuclear Force ." ©2015 College Hill Coaching.As any good contractor will tell you, a soundstructure requires stable materials. But atoms,the building blocks of everything we know andlove—bunnies, brownies, and best friends—(5) dont appear to be models of stability. Why aresome atoms, like sodium, so hyperactive whileothers, like helium, are so aloof? Why do theelectrons that inhabit atoms jump around sostrangely, from one bizarrely shaped orbital to(10) another? And why do protons, the bits thatgive atoms their heft and personality, sticktogether at all?We are told that every atom has a tinynucleus containing positively charged protons(15) and uncharged neutrons, swarmed by a cloudof speedy electrons. We are also told that likecharges, such as protons, repel each other witha force that shoots up to infinity as they getcloser. Even worse, you cant get much closer(20) than two protons in the nucleus of an atom. Sowhats keeping atomic nuclei from flying apart?Obviously, some other force must be at workinside the atom, something that we cant detect atour human scale. Physicists call this the(25) “strong nuclear force.” But where does itcome from?In order for this force to account for thebinding of protons in the nucleus, it must havecertain interesting features. First, it cant have any(30) sizeable effect beyond the radius of the atom itself,or it would play havoc with the nuclei of adjacentatoms, destroying matter as we know it. Second,it must perfectly balance the repulsive force ofelectricity at an “equilibrium point” of about(35) 0.7 x 10-15 meters, the average distance betweenbound protons, in order to create a stable nucleus.Third, it must repel at even shorter distances, orelse neutrons (which dont have any electrostaticrepulsion to balance the strong nuclear force)(40) would collapse into each other. The graphshows the behavior of such a force relativeto the repulsive electrostatic force.In 1935, Japanese physicist Hideki Yukawaproposed that the nuclear force was conveyed by(45) a then-undiscovered heavy subatomic particlehe called the pi meson (or “pion”), which (unlikethe photon, which conveys the electrostaticforce) decays very quickly and therefore conveys apowerful force only over a very short distance.(50) Professor Yukawas theory, however, wasdealt a mortal blow by a series of experimentsconducted at Los Alamos National Laboratoryin the early 1990s that demonstrated that pionscarry force only over distances greater than the(55) distance between bound protons. The pion was aplumbers wrench trying to do a tweezers job.Current atomic theory suggests that thestrong nuclear force is most likely conveyed bymassless particles called “gluons” according(60) to the theory of quantum chromodynamics, orQCD for short. According to QCD, protons andneutrons are composed of smaller particlescalled quarks, which are held together by theaptly named gluons. This quark-binding force has(65) a “residue” that extends beyond the protons andneutrons themselves to provide just enough forceto bind the protons and neutrons together.If youre hoping that QCD ties up atomicbehavior with a tidy little bow, you may be just(70) a bit disappointed. As a quantum theory, itconceives of space and time as tiny chunks thatoccasionally misbehave, rather than smoothpredictable quantities, and its mathematicalformulas are perhaps as hard to penetrate as the(75) nucleus itself.Q.Which of the following best describes the structure of the passage as a whole?a)a series of intuitive illustrations of a complex physical theoryb)a description of a technical puzzle and the attempts to solve itc)an account of an experimental finding and its surprising implicationsd)a historical over view of a heated scientific controversyCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice SAT tests.

|
Explore Courses for SAT exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























