ACT Exam > ACT Questions > Directions:Read the passages and choose the b...
Start Learning for Free
Directions: Read the passages and choose the best answer to each question.
Passage
The Great Lakes—Huron, Ontario, Michigan, Erie, and Superior—form the largest freshwater system in the world.
Each of the lakes tends to stratify, or form layers of warmer and colder water, depending on the season. This is called seasonal turnover. In winter, for example, the coldest water in the lake lies just below the surface ice. The water gets progressively warmer at deeper levels. In spring, the sun melts the ice, and the surface water warms. Because the surface water is still cooler than the layers below, the water at the surface sinks to the bottom of the lake, forcing the cooler water at the bottom of the lake to the surface. This mixing, known as spring turnover, eliminates the temperature stratification that was established during the winter.
In the absence of this thermal layering, wind continues to mix the water to a greater depth, bringing oxygen (O2) to the bottom of the lake and nutrients to the surface. This results in a relatively even distribution of O2 throughout the lake. When summer arrives, the lake again becomes stratified, with warm water at the surface, and cold water at the bottom. A narrow zone of water undergoing rapid temperature changes separates these layers. This zone is called the thermocline. Cool, fall temperatures cause the lake water to mix again, until the surface begins to freeze and the winter stratification is reestablished.
The stability of the lake’s stratification depends on several factors: the lake’s depth, shape, and size, as well as the wind and both the inflow and outflow of lake water. Lakes with a lot of water flowing into and out of them do not develop consistent and lasting thermal stratification.
Passage
The Great Lakes—Huron, Ontario, Michigan, Erie, and Superior—form the largest freshwater system in the world.
Each of the lakes tends to stratify, or form layers of warmer and colder water, depending on the season. This is called seasonal turnover. In winter, for example, the coldest water in the lake lies just below the surface ice. The water gets progressively warmer at deeper levels. In spring, the sun melts the ice, and the surface water warms. Because the surface water is still cooler than the layers below, the water at the surface sinks to the bottom of the lake, forcing the cooler water at the bottom of the lake to the surface. This mixing, known as spring turnover, eliminates the temperature stratification that was established during the winter.
In the absence of this thermal layering, wind continues to mix the water to a greater depth, bringing oxygen (O2) to the bottom of the lake and nutrients to the surface. This results in a relatively even distribution of O2 throughout the lake. When summer arrives, the lake again becomes stratified, with warm water at the surface, and cold water at the bottom. A narrow zone of water undergoing rapid temperature changes separates these layers. This zone is called the thermocline. Cool, fall temperatures cause the lake water to mix again, until the surface begins to freeze and the winter stratification is reestablished.
The stability of the lake’s stratification depends on several factors: the lake’s depth, shape, and size, as well as the wind and both the inflow and outflow of lake water. Lakes with a lot of water flowing into and out of them do not develop consistent and lasting thermal stratification.

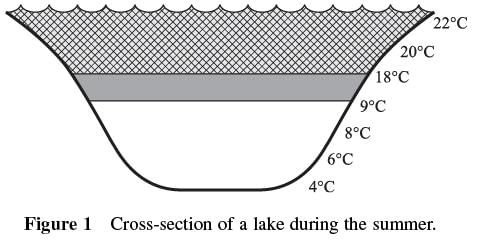
Q. According to Figure 1, the temperature of the water below the thermocline is:
- a)higher than the temperature of the water above the thermocline.
- b)equal to the temperature of the water above the thermocline.
- c)lower than the temperature of the water above the thermocline.
- d)equal to the average temperature of the water in the lake.
Correct answer is option 'C'. Can you explain this answer?
Most Upvoted Answer
Directions:Read the passages and choose the best answer to each questi...
The best answer is c. Figure 1 shows that the temperatures near the bottom of the lake are lower than the temperatures near the surface of the lake. The thermocline is shown as a layer separating the surface water from the deeper water. This best supports answer choice c.

|
Explore Courses for ACT exam
|

|
Similar ACT Doubts
Directions:Read the passages and choose the best answer to each question.PassageThe Great Lakes—Huron, Ontario, Michigan, Erie, and Superior—form the largest freshwater system in the world.Each of the lakes tends to stratify, or form layers of warmer and colder water, depending on the season. This is called seasonal turnover. In winter, for example, the coldest water in the lake lies just below the surface ice. The water gets progressively warmer at deeper levels. In spring, the sun melts the ice, and the surface water warms. Because the surface water is still cooler than the layers below, the water at the surface sinks to the bottom of the lake, forcing the cooler water at the bottom of the lake to the surface. This mixing, known as spring turnover, eliminates the temperature stratification that was established during the winter.In the absence of this thermal layering, wind continues to mix the water to a greater depth, bringing oxygen (O2) to the bottom of the lake and nutrients to the surface. This results in a relatively even distribution of O2 throughout the lake. When summer arrives, the lake again becomes stratified, with warm water at the surface, and cold water at the bottom. A narrow zone of water undergoing rapid temperature changes separates these layers. This zone is called the thermocline. Cool, fall temperatures cause the lake water to mix again, until the surface begins to freeze and the winter stratification is reestablished.The stability of the lake’s stratification depends on several factors: the lake’s depth, shape, and size, as well as the wind and both the inflow and outflow of lake water. Lakes with a lot of water flowing into and out of them do not develop consistent and lasting thermal stratification.Q. According to Figure 1, the temperature of the water below the thermocline is:a)higher than the temperature of the water above the thermocline.b)equal to the temperature of the water above the thermocline.c)lower than the temperature of the water above the thermocline.d)equal to the average temperature of the water in the lake.Correct answer is option 'C'. Can you explain this answer?
Question Description
Directions:Read the passages and choose the best answer to each question.PassageThe Great Lakes—Huron, Ontario, Michigan, Erie, and Superior—form the largest freshwater system in the world.Each of the lakes tends to stratify, or form layers of warmer and colder water, depending on the season. This is called seasonal turnover. In winter, for example, the coldest water in the lake lies just below the surface ice. The water gets progressively warmer at deeper levels. In spring, the sun melts the ice, and the surface water warms. Because the surface water is still cooler than the layers below, the water at the surface sinks to the bottom of the lake, forcing the cooler water at the bottom of the lake to the surface. This mixing, known as spring turnover, eliminates the temperature stratification that was established during the winter.In the absence of this thermal layering, wind continues to mix the water to a greater depth, bringing oxygen (O2) to the bottom of the lake and nutrients to the surface. This results in a relatively even distribution of O2 throughout the lake. When summer arrives, the lake again becomes stratified, with warm water at the surface, and cold water at the bottom. A narrow zone of water undergoing rapid temperature changes separates these layers. This zone is called the thermocline. Cool, fall temperatures cause the lake water to mix again, until the surface begins to freeze and the winter stratification is reestablished.The stability of the lake’s stratification depends on several factors: the lake’s depth, shape, and size, as well as the wind and both the inflow and outflow of lake water. Lakes with a lot of water flowing into and out of them do not develop consistent and lasting thermal stratification.Q. According to Figure 1, the temperature of the water below the thermocline is:a)higher than the temperature of the water above the thermocline.b)equal to the temperature of the water above the thermocline.c)lower than the temperature of the water above the thermocline.d)equal to the average temperature of the water in the lake.Correct answer is option 'C'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Directions:Read the passages and choose the best answer to each question.PassageThe Great Lakes—Huron, Ontario, Michigan, Erie, and Superior—form the largest freshwater system in the world.Each of the lakes tends to stratify, or form layers of warmer and colder water, depending on the season. This is called seasonal turnover. In winter, for example, the coldest water in the lake lies just below the surface ice. The water gets progressively warmer at deeper levels. In spring, the sun melts the ice, and the surface water warms. Because the surface water is still cooler than the layers below, the water at the surface sinks to the bottom of the lake, forcing the cooler water at the bottom of the lake to the surface. This mixing, known as spring turnover, eliminates the temperature stratification that was established during the winter.In the absence of this thermal layering, wind continues to mix the water to a greater depth, bringing oxygen (O2) to the bottom of the lake and nutrients to the surface. This results in a relatively even distribution of O2 throughout the lake. When summer arrives, the lake again becomes stratified, with warm water at the surface, and cold water at the bottom. A narrow zone of water undergoing rapid temperature changes separates these layers. This zone is called the thermocline. Cool, fall temperatures cause the lake water to mix again, until the surface begins to freeze and the winter stratification is reestablished.The stability of the lake’s stratification depends on several factors: the lake’s depth, shape, and size, as well as the wind and both the inflow and outflow of lake water. Lakes with a lot of water flowing into and out of them do not develop consistent and lasting thermal stratification.Q. According to Figure 1, the temperature of the water below the thermocline is:a)higher than the temperature of the water above the thermocline.b)equal to the temperature of the water above the thermocline.c)lower than the temperature of the water above the thermocline.d)equal to the average temperature of the water in the lake.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions:Read the passages and choose the best answer to each question.PassageThe Great Lakes—Huron, Ontario, Michigan, Erie, and Superior—form the largest freshwater system in the world.Each of the lakes tends to stratify, or form layers of warmer and colder water, depending on the season. This is called seasonal turnover. In winter, for example, the coldest water in the lake lies just below the surface ice. The water gets progressively warmer at deeper levels. In spring, the sun melts the ice, and the surface water warms. Because the surface water is still cooler than the layers below, the water at the surface sinks to the bottom of the lake, forcing the cooler water at the bottom of the lake to the surface. This mixing, known as spring turnover, eliminates the temperature stratification that was established during the winter.In the absence of this thermal layering, wind continues to mix the water to a greater depth, bringing oxygen (O2) to the bottom of the lake and nutrients to the surface. This results in a relatively even distribution of O2 throughout the lake. When summer arrives, the lake again becomes stratified, with warm water at the surface, and cold water at the bottom. A narrow zone of water undergoing rapid temperature changes separates these layers. This zone is called the thermocline. Cool, fall temperatures cause the lake water to mix again, until the surface begins to freeze and the winter stratification is reestablished.The stability of the lake’s stratification depends on several factors: the lake’s depth, shape, and size, as well as the wind and both the inflow and outflow of lake water. Lakes with a lot of water flowing into and out of them do not develop consistent and lasting thermal stratification.Q. According to Figure 1, the temperature of the water below the thermocline is:a)higher than the temperature of the water above the thermocline.b)equal to the temperature of the water above the thermocline.c)lower than the temperature of the water above the thermocline.d)equal to the average temperature of the water in the lake.Correct answer is option 'C'. Can you explain this answer?.
Directions:Read the passages and choose the best answer to each question.PassageThe Great Lakes—Huron, Ontario, Michigan, Erie, and Superior—form the largest freshwater system in the world.Each of the lakes tends to stratify, or form layers of warmer and colder water, depending on the season. This is called seasonal turnover. In winter, for example, the coldest water in the lake lies just below the surface ice. The water gets progressively warmer at deeper levels. In spring, the sun melts the ice, and the surface water warms. Because the surface water is still cooler than the layers below, the water at the surface sinks to the bottom of the lake, forcing the cooler water at the bottom of the lake to the surface. This mixing, known as spring turnover, eliminates the temperature stratification that was established during the winter.In the absence of this thermal layering, wind continues to mix the water to a greater depth, bringing oxygen (O2) to the bottom of the lake and nutrients to the surface. This results in a relatively even distribution of O2 throughout the lake. When summer arrives, the lake again becomes stratified, with warm water at the surface, and cold water at the bottom. A narrow zone of water undergoing rapid temperature changes separates these layers. This zone is called the thermocline. Cool, fall temperatures cause the lake water to mix again, until the surface begins to freeze and the winter stratification is reestablished.The stability of the lake’s stratification depends on several factors: the lake’s depth, shape, and size, as well as the wind and both the inflow and outflow of lake water. Lakes with a lot of water flowing into and out of them do not develop consistent and lasting thermal stratification.Q. According to Figure 1, the temperature of the water below the thermocline is:a)higher than the temperature of the water above the thermocline.b)equal to the temperature of the water above the thermocline.c)lower than the temperature of the water above the thermocline.d)equal to the average temperature of the water in the lake.Correct answer is option 'C'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Directions:Read the passages and choose the best answer to each question.PassageThe Great Lakes—Huron, Ontario, Michigan, Erie, and Superior—form the largest freshwater system in the world.Each of the lakes tends to stratify, or form layers of warmer and colder water, depending on the season. This is called seasonal turnover. In winter, for example, the coldest water in the lake lies just below the surface ice. The water gets progressively warmer at deeper levels. In spring, the sun melts the ice, and the surface water warms. Because the surface water is still cooler than the layers below, the water at the surface sinks to the bottom of the lake, forcing the cooler water at the bottom of the lake to the surface. This mixing, known as spring turnover, eliminates the temperature stratification that was established during the winter.In the absence of this thermal layering, wind continues to mix the water to a greater depth, bringing oxygen (O2) to the bottom of the lake and nutrients to the surface. This results in a relatively even distribution of O2 throughout the lake. When summer arrives, the lake again becomes stratified, with warm water at the surface, and cold water at the bottom. A narrow zone of water undergoing rapid temperature changes separates these layers. This zone is called the thermocline. Cool, fall temperatures cause the lake water to mix again, until the surface begins to freeze and the winter stratification is reestablished.The stability of the lake’s stratification depends on several factors: the lake’s depth, shape, and size, as well as the wind and both the inflow and outflow of lake water. Lakes with a lot of water flowing into and out of them do not develop consistent and lasting thermal stratification.Q. According to Figure 1, the temperature of the water below the thermocline is:a)higher than the temperature of the water above the thermocline.b)equal to the temperature of the water above the thermocline.c)lower than the temperature of the water above the thermocline.d)equal to the average temperature of the water in the lake.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions:Read the passages and choose the best answer to each question.PassageThe Great Lakes—Huron, Ontario, Michigan, Erie, and Superior—form the largest freshwater system in the world.Each of the lakes tends to stratify, or form layers of warmer and colder water, depending on the season. This is called seasonal turnover. In winter, for example, the coldest water in the lake lies just below the surface ice. The water gets progressively warmer at deeper levels. In spring, the sun melts the ice, and the surface water warms. Because the surface water is still cooler than the layers below, the water at the surface sinks to the bottom of the lake, forcing the cooler water at the bottom of the lake to the surface. This mixing, known as spring turnover, eliminates the temperature stratification that was established during the winter.In the absence of this thermal layering, wind continues to mix the water to a greater depth, bringing oxygen (O2) to the bottom of the lake and nutrients to the surface. This results in a relatively even distribution of O2 throughout the lake. When summer arrives, the lake again becomes stratified, with warm water at the surface, and cold water at the bottom. A narrow zone of water undergoing rapid temperature changes separates these layers. This zone is called the thermocline. Cool, fall temperatures cause the lake water to mix again, until the surface begins to freeze and the winter stratification is reestablished.The stability of the lake’s stratification depends on several factors: the lake’s depth, shape, and size, as well as the wind and both the inflow and outflow of lake water. Lakes with a lot of water flowing into and out of them do not develop consistent and lasting thermal stratification.Q. According to Figure 1, the temperature of the water below the thermocline is:a)higher than the temperature of the water above the thermocline.b)equal to the temperature of the water above the thermocline.c)lower than the temperature of the water above the thermocline.d)equal to the average temperature of the water in the lake.Correct answer is option 'C'. Can you explain this answer?.
Solutions for Directions:Read the passages and choose the best answer to each question.PassageThe Great Lakes—Huron, Ontario, Michigan, Erie, and Superior—form the largest freshwater system in the world.Each of the lakes tends to stratify, or form layers of warmer and colder water, depending on the season. This is called seasonal turnover. In winter, for example, the coldest water in the lake lies just below the surface ice. The water gets progressively warmer at deeper levels. In spring, the sun melts the ice, and the surface water warms. Because the surface water is still cooler than the layers below, the water at the surface sinks to the bottom of the lake, forcing the cooler water at the bottom of the lake to the surface. This mixing, known as spring turnover, eliminates the temperature stratification that was established during the winter.In the absence of this thermal layering, wind continues to mix the water to a greater depth, bringing oxygen (O2) to the bottom of the lake and nutrients to the surface. This results in a relatively even distribution of O2 throughout the lake. When summer arrives, the lake again becomes stratified, with warm water at the surface, and cold water at the bottom. A narrow zone of water undergoing rapid temperature changes separates these layers. This zone is called the thermocline. Cool, fall temperatures cause the lake water to mix again, until the surface begins to freeze and the winter stratification is reestablished.The stability of the lake’s stratification depends on several factors: the lake’s depth, shape, and size, as well as the wind and both the inflow and outflow of lake water. Lakes with a lot of water flowing into and out of them do not develop consistent and lasting thermal stratification.Q. According to Figure 1, the temperature of the water below the thermocline is:a)higher than the temperature of the water above the thermocline.b)equal to the temperature of the water above the thermocline.c)lower than the temperature of the water above the thermocline.d)equal to the average temperature of the water in the lake.Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for ACT.
Download more important topics, notes, lectures and mock test series for ACT Exam by signing up for free.
Here you can find the meaning of Directions:Read the passages and choose the best answer to each question.PassageThe Great Lakes—Huron, Ontario, Michigan, Erie, and Superior—form the largest freshwater system in the world.Each of the lakes tends to stratify, or form layers of warmer and colder water, depending on the season. This is called seasonal turnover. In winter, for example, the coldest water in the lake lies just below the surface ice. The water gets progressively warmer at deeper levels. In spring, the sun melts the ice, and the surface water warms. Because the surface water is still cooler than the layers below, the water at the surface sinks to the bottom of the lake, forcing the cooler water at the bottom of the lake to the surface. This mixing, known as spring turnover, eliminates the temperature stratification that was established during the winter.In the absence of this thermal layering, wind continues to mix the water to a greater depth, bringing oxygen (O2) to the bottom of the lake and nutrients to the surface. This results in a relatively even distribution of O2 throughout the lake. When summer arrives, the lake again becomes stratified, with warm water at the surface, and cold water at the bottom. A narrow zone of water undergoing rapid temperature changes separates these layers. This zone is called the thermocline. Cool, fall temperatures cause the lake water to mix again, until the surface begins to freeze and the winter stratification is reestablished.The stability of the lake’s stratification depends on several factors: the lake’s depth, shape, and size, as well as the wind and both the inflow and outflow of lake water. Lakes with a lot of water flowing into and out of them do not develop consistent and lasting thermal stratification.Q. According to Figure 1, the temperature of the water below the thermocline is:a)higher than the temperature of the water above the thermocline.b)equal to the temperature of the water above the thermocline.c)lower than the temperature of the water above the thermocline.d)equal to the average temperature of the water in the lake.Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Directions:Read the passages and choose the best answer to each question.PassageThe Great Lakes—Huron, Ontario, Michigan, Erie, and Superior—form the largest freshwater system in the world.Each of the lakes tends to stratify, or form layers of warmer and colder water, depending on the season. This is called seasonal turnover. In winter, for example, the coldest water in the lake lies just below the surface ice. The water gets progressively warmer at deeper levels. In spring, the sun melts the ice, and the surface water warms. Because the surface water is still cooler than the layers below, the water at the surface sinks to the bottom of the lake, forcing the cooler water at the bottom of the lake to the surface. This mixing, known as spring turnover, eliminates the temperature stratification that was established during the winter.In the absence of this thermal layering, wind continues to mix the water to a greater depth, bringing oxygen (O2) to the bottom of the lake and nutrients to the surface. This results in a relatively even distribution of O2 throughout the lake. When summer arrives, the lake again becomes stratified, with warm water at the surface, and cold water at the bottom. A narrow zone of water undergoing rapid temperature changes separates these layers. This zone is called the thermocline. Cool, fall temperatures cause the lake water to mix again, until the surface begins to freeze and the winter stratification is reestablished.The stability of the lake’s stratification depends on several factors: the lake’s depth, shape, and size, as well as the wind and both the inflow and outflow of lake water. Lakes with a lot of water flowing into and out of them do not develop consistent and lasting thermal stratification.Q. According to Figure 1, the temperature of the water below the thermocline is:a)higher than the temperature of the water above the thermocline.b)equal to the temperature of the water above the thermocline.c)lower than the temperature of the water above the thermocline.d)equal to the average temperature of the water in the lake.Correct answer is option 'C'. Can you explain this answer?, a detailed solution for Directions:Read the passages and choose the best answer to each question.PassageThe Great Lakes—Huron, Ontario, Michigan, Erie, and Superior—form the largest freshwater system in the world.Each of the lakes tends to stratify, or form layers of warmer and colder water, depending on the season. This is called seasonal turnover. In winter, for example, the coldest water in the lake lies just below the surface ice. The water gets progressively warmer at deeper levels. In spring, the sun melts the ice, and the surface water warms. Because the surface water is still cooler than the layers below, the water at the surface sinks to the bottom of the lake, forcing the cooler water at the bottom of the lake to the surface. This mixing, known as spring turnover, eliminates the temperature stratification that was established during the winter.In the absence of this thermal layering, wind continues to mix the water to a greater depth, bringing oxygen (O2) to the bottom of the lake and nutrients to the surface. This results in a relatively even distribution of O2 throughout the lake. When summer arrives, the lake again becomes stratified, with warm water at the surface, and cold water at the bottom. A narrow zone of water undergoing rapid temperature changes separates these layers. This zone is called the thermocline. Cool, fall temperatures cause the lake water to mix again, until the surface begins to freeze and the winter stratification is reestablished.The stability of the lake’s stratification depends on several factors: the lake’s depth, shape, and size, as well as the wind and both the inflow and outflow of lake water. Lakes with a lot of water flowing into and out of them do not develop consistent and lasting thermal stratification.Q. According to Figure 1, the temperature of the water below the thermocline is:a)higher than the temperature of the water above the thermocline.b)equal to the temperature of the water above the thermocline.c)lower than the temperature of the water above the thermocline.d)equal to the average temperature of the water in the lake.Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of Directions:Read the passages and choose the best answer to each question.PassageThe Great Lakes—Huron, Ontario, Michigan, Erie, and Superior—form the largest freshwater system in the world.Each of the lakes tends to stratify, or form layers of warmer and colder water, depending on the season. This is called seasonal turnover. In winter, for example, the coldest water in the lake lies just below the surface ice. The water gets progressively warmer at deeper levels. In spring, the sun melts the ice, and the surface water warms. Because the surface water is still cooler than the layers below, the water at the surface sinks to the bottom of the lake, forcing the cooler water at the bottom of the lake to the surface. This mixing, known as spring turnover, eliminates the temperature stratification that was established during the winter.In the absence of this thermal layering, wind continues to mix the water to a greater depth, bringing oxygen (O2) to the bottom of the lake and nutrients to the surface. This results in a relatively even distribution of O2 throughout the lake. When summer arrives, the lake again becomes stratified, with warm water at the surface, and cold water at the bottom. A narrow zone of water undergoing rapid temperature changes separates these layers. This zone is called the thermocline. Cool, fall temperatures cause the lake water to mix again, until the surface begins to freeze and the winter stratification is reestablished.The stability of the lake’s stratification depends on several factors: the lake’s depth, shape, and size, as well as the wind and both the inflow and outflow of lake water. Lakes with a lot of water flowing into and out of them do not develop consistent and lasting thermal stratification.Q. According to Figure 1, the temperature of the water below the thermocline is:a)higher than the temperature of the water above the thermocline.b)equal to the temperature of the water above the thermocline.c)lower than the temperature of the water above the thermocline.d)equal to the average temperature of the water in the lake.Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Directions:Read the passages and choose the best answer to each question.PassageThe Great Lakes—Huron, Ontario, Michigan, Erie, and Superior—form the largest freshwater system in the world.Each of the lakes tends to stratify, or form layers of warmer and colder water, depending on the season. This is called seasonal turnover. In winter, for example, the coldest water in the lake lies just below the surface ice. The water gets progressively warmer at deeper levels. In spring, the sun melts the ice, and the surface water warms. Because the surface water is still cooler than the layers below, the water at the surface sinks to the bottom of the lake, forcing the cooler water at the bottom of the lake to the surface. This mixing, known as spring turnover, eliminates the temperature stratification that was established during the winter.In the absence of this thermal layering, wind continues to mix the water to a greater depth, bringing oxygen (O2) to the bottom of the lake and nutrients to the surface. This results in a relatively even distribution of O2 throughout the lake. When summer arrives, the lake again becomes stratified, with warm water at the surface, and cold water at the bottom. A narrow zone of water undergoing rapid temperature changes separates these layers. This zone is called the thermocline. Cool, fall temperatures cause the lake water to mix again, until the surface begins to freeze and the winter stratification is reestablished.The stability of the lake’s stratification depends on several factors: the lake’s depth, shape, and size, as well as the wind and both the inflow and outflow of lake water. Lakes with a lot of water flowing into and out of them do not develop consistent and lasting thermal stratification.Q. According to Figure 1, the temperature of the water below the thermocline is:a)higher than the temperature of the water above the thermocline.b)equal to the temperature of the water above the thermocline.c)lower than the temperature of the water above the thermocline.d)equal to the average temperature of the water in the lake.Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice ACT tests.

|
Explore Courses for ACT exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























