ACT Exam > ACT Questions > Directions:Read the passages and choose the b...
Start Learning for Free
Directions: Read the passages and choose the best answer to each question.
Passage
Certain preservatives known as sulfites are often added to fruit products to keep the fruit fresher longer. Use of sulfites is controversial because studies have linked sulfites to severe reactions in some asthmatics. Students performed 2 experiments to measure sulfite levels.
Experiment 1
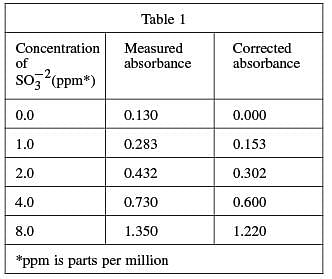
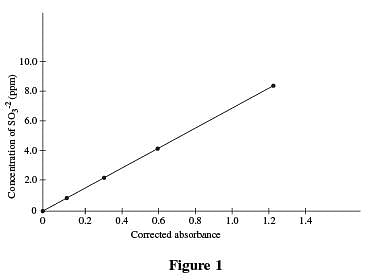
Four solutions, each containing a different amount of sulfite dissolved in H2 O were prepared. A coloring agent was added that binds with sulfite to form a red compound that strongly absorbs light of a specific wavelength, and each solution was diluted to 100 mL. A blank solution was prepared in the same manner, but no sulfite was added. A colorimeter (a device that measures how much light of a selected wavelength is absorbed by a sample) was used to measure the absorbance of each solution. The absorbances were corrected by subtracting the absorbance of the blank solution from each reading (see Table 1 and Figure 1).
Experiment 2
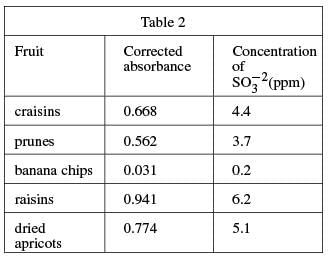
A 100 g fruit sample was ground in a food processor with 50 mL of H2O and the mixture was filtered. The food processor and remaining fruit were then washed with H2O, these washings were filtered, and the liquid was added to the sample solution. The coloring agent was added and the solution was diluted to 100 mL. The procedure was repeated for several fruits, and the absorbances were measured (see Table 2).
Certain preservatives known as sulfites are often added to fruit products to keep the fruit fresher longer. Use of sulfites is controversial because studies have linked sulfites to severe reactions in some asthmatics. Students performed 2 experiments to measure sulfite levels.
Experiment 1
Four solutions, each containing a different amount of sulfite dissolved in H2 O were prepared. A coloring agent was added that binds with sulfite to form a red compound that strongly absorbs light of a specific wavelength, and each solution was diluted to 100 mL. A blank solution was prepared in the same manner, but no sulfite was added. A colorimeter (a device that measures how much light of a selected wavelength is absorbed by a sample) was used to measure the absorbance of each solution. The absorbances were corrected by subtracting the absorbance of the blank solution from each reading (see Table 1 and Figure 1).


Experiment 2
A 100 g fruit sample was ground in a food processor with 50 mL of H2O and the mixture was filtered. The food processor and remaining fruit were then washed with H2O, these washings were filtered, and the liquid was added to the sample solution. The coloring agent was added and the solution was diluted to 100 mL. The procedure was repeated for several fruits, and the absorbances were measured (see Table 2).

Q. If Experiments 1 and 2 were repeated using a different coloring agent that produces a different color when it binds with sulfite, which of the following changes in procedure would be necessary?
- a)The new coloring agent should be added to the blank solution, but not to the sample solutions.
- b)Both of the coloring agents should be added to the blank solution and to all of the samples.
- c)The absorbance of the blank solution made with the new coloring agent should be added to the measured absorbances.
- d)The colorimeter should be set to measure at a different wavelength of light.
Correct answer is option 'D'. Can you explain this answer?
Most Upvoted Answer
Directions:Read the passages and choose the best answer to each questi...
According to the passage, a colorimeter is a device that measures how much light of a selected wavelength is absorbed. Because different colors have different wavelengths, the colorimeter needs to be adjusted to read at its maximum capacity. The other answer choices are not supported by the passage.

|
Explore Courses for ACT exam
|

|
Directions:Read the passages and choose the best answer to each question.PassageCertain preservatives known as sulfites are often added to fruit products to keep the fruit fresher longer. Use of sulfites is controversial because studies have linked sulfites to severe reactions in some asthmatics. Students performed 2 experiments to measure sulfite levels.Experiment 1Four solutions, each containing a different amount of sulfite dissolved in H2 O were prepared. A coloring agent was added that binds with sulfite to form a red compound that strongly absorbs light of a specific wavelength, and each solution was diluted to 100 mL. A blank solution was prepared in the same manner, but no sulfite was added. A colorimeter (a device that measures how much light of a selected wavelength is absorbed by a sample) was used to measure the absorbance of each solution. The absorbances were corrected by subtracting the absorbance of the blank solution from each reading (see Table 1 and Figure 1).Experiment 2A 100 g fruit sample was ground in a food processor with 50 mL of H2O and the mixture was filtered. The food processor and remaining fruit were then washed with H2O, these washings were filtered, and the liquid was added to the sample solution. The coloring agent was added and the solution was diluted to 100 mL. The procedure was repeated for several fruits, and the absorbances were measured (see Table 2).Q.If Experiments 1 and 2 were repeated using a different coloring agent that produces a different color when it binds with sulfite, which of the following changes in procedure would be necessary?a)The new coloring agent should be added to the blank solution, but not to the sample solutions.b)Both of the coloring agents should be added to the blank solution and to all of the samples.c)The absorbance of the blank solution made with the new coloring agent should be added to the measured absorbances.d)The colorimeter should be set to measure at a different wavelength of light.Correct answer is option 'D'. Can you explain this answer?
Question Description
Directions:Read the passages and choose the best answer to each question.PassageCertain preservatives known as sulfites are often added to fruit products to keep the fruit fresher longer. Use of sulfites is controversial because studies have linked sulfites to severe reactions in some asthmatics. Students performed 2 experiments to measure sulfite levels.Experiment 1Four solutions, each containing a different amount of sulfite dissolved in H2 O were prepared. A coloring agent was added that binds with sulfite to form a red compound that strongly absorbs light of a specific wavelength, and each solution was diluted to 100 mL. A blank solution was prepared in the same manner, but no sulfite was added. A colorimeter (a device that measures how much light of a selected wavelength is absorbed by a sample) was used to measure the absorbance of each solution. The absorbances were corrected by subtracting the absorbance of the blank solution from each reading (see Table 1 and Figure 1).Experiment 2A 100 g fruit sample was ground in a food processor with 50 mL of H2O and the mixture was filtered. The food processor and remaining fruit were then washed with H2O, these washings were filtered, and the liquid was added to the sample solution. The coloring agent was added and the solution was diluted to 100 mL. The procedure was repeated for several fruits, and the absorbances were measured (see Table 2).Q.If Experiments 1 and 2 were repeated using a different coloring agent that produces a different color when it binds with sulfite, which of the following changes in procedure would be necessary?a)The new coloring agent should be added to the blank solution, but not to the sample solutions.b)Both of the coloring agents should be added to the blank solution and to all of the samples.c)The absorbance of the blank solution made with the new coloring agent should be added to the measured absorbances.d)The colorimeter should be set to measure at a different wavelength of light.Correct answer is option 'D'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Directions:Read the passages and choose the best answer to each question.PassageCertain preservatives known as sulfites are often added to fruit products to keep the fruit fresher longer. Use of sulfites is controversial because studies have linked sulfites to severe reactions in some asthmatics. Students performed 2 experiments to measure sulfite levels.Experiment 1Four solutions, each containing a different amount of sulfite dissolved in H2 O were prepared. A coloring agent was added that binds with sulfite to form a red compound that strongly absorbs light of a specific wavelength, and each solution was diluted to 100 mL. A blank solution was prepared in the same manner, but no sulfite was added. A colorimeter (a device that measures how much light of a selected wavelength is absorbed by a sample) was used to measure the absorbance of each solution. The absorbances were corrected by subtracting the absorbance of the blank solution from each reading (see Table 1 and Figure 1).Experiment 2A 100 g fruit sample was ground in a food processor with 50 mL of H2O and the mixture was filtered. The food processor and remaining fruit were then washed with H2O, these washings were filtered, and the liquid was added to the sample solution. The coloring agent was added and the solution was diluted to 100 mL. The procedure was repeated for several fruits, and the absorbances were measured (see Table 2).Q.If Experiments 1 and 2 were repeated using a different coloring agent that produces a different color when it binds with sulfite, which of the following changes in procedure would be necessary?a)The new coloring agent should be added to the blank solution, but not to the sample solutions.b)Both of the coloring agents should be added to the blank solution and to all of the samples.c)The absorbance of the blank solution made with the new coloring agent should be added to the measured absorbances.d)The colorimeter should be set to measure at a different wavelength of light.Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions:Read the passages and choose the best answer to each question.PassageCertain preservatives known as sulfites are often added to fruit products to keep the fruit fresher longer. Use of sulfites is controversial because studies have linked sulfites to severe reactions in some asthmatics. Students performed 2 experiments to measure sulfite levels.Experiment 1Four solutions, each containing a different amount of sulfite dissolved in H2 O were prepared. A coloring agent was added that binds with sulfite to form a red compound that strongly absorbs light of a specific wavelength, and each solution was diluted to 100 mL. A blank solution was prepared in the same manner, but no sulfite was added. A colorimeter (a device that measures how much light of a selected wavelength is absorbed by a sample) was used to measure the absorbance of each solution. The absorbances were corrected by subtracting the absorbance of the blank solution from each reading (see Table 1 and Figure 1).Experiment 2A 100 g fruit sample was ground in a food processor with 50 mL of H2O and the mixture was filtered. The food processor and remaining fruit were then washed with H2O, these washings were filtered, and the liquid was added to the sample solution. The coloring agent was added and the solution was diluted to 100 mL. The procedure was repeated for several fruits, and the absorbances were measured (see Table 2).Q.If Experiments 1 and 2 were repeated using a different coloring agent that produces a different color when it binds with sulfite, which of the following changes in procedure would be necessary?a)The new coloring agent should be added to the blank solution, but not to the sample solutions.b)Both of the coloring agents should be added to the blank solution and to all of the samples.c)The absorbance of the blank solution made with the new coloring agent should be added to the measured absorbances.d)The colorimeter should be set to measure at a different wavelength of light.Correct answer is option 'D'. Can you explain this answer?.
Directions:Read the passages and choose the best answer to each question.PassageCertain preservatives known as sulfites are often added to fruit products to keep the fruit fresher longer. Use of sulfites is controversial because studies have linked sulfites to severe reactions in some asthmatics. Students performed 2 experiments to measure sulfite levels.Experiment 1Four solutions, each containing a different amount of sulfite dissolved in H2 O were prepared. A coloring agent was added that binds with sulfite to form a red compound that strongly absorbs light of a specific wavelength, and each solution was diluted to 100 mL. A blank solution was prepared in the same manner, but no sulfite was added. A colorimeter (a device that measures how much light of a selected wavelength is absorbed by a sample) was used to measure the absorbance of each solution. The absorbances were corrected by subtracting the absorbance of the blank solution from each reading (see Table 1 and Figure 1).Experiment 2A 100 g fruit sample was ground in a food processor with 50 mL of H2O and the mixture was filtered. The food processor and remaining fruit were then washed with H2O, these washings were filtered, and the liquid was added to the sample solution. The coloring agent was added and the solution was diluted to 100 mL. The procedure was repeated for several fruits, and the absorbances were measured (see Table 2).Q.If Experiments 1 and 2 were repeated using a different coloring agent that produces a different color when it binds with sulfite, which of the following changes in procedure would be necessary?a)The new coloring agent should be added to the blank solution, but not to the sample solutions.b)Both of the coloring agents should be added to the blank solution and to all of the samples.c)The absorbance of the blank solution made with the new coloring agent should be added to the measured absorbances.d)The colorimeter should be set to measure at a different wavelength of light.Correct answer is option 'D'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Directions:Read the passages and choose the best answer to each question.PassageCertain preservatives known as sulfites are often added to fruit products to keep the fruit fresher longer. Use of sulfites is controversial because studies have linked sulfites to severe reactions in some asthmatics. Students performed 2 experiments to measure sulfite levels.Experiment 1Four solutions, each containing a different amount of sulfite dissolved in H2 O were prepared. A coloring agent was added that binds with sulfite to form a red compound that strongly absorbs light of a specific wavelength, and each solution was diluted to 100 mL. A blank solution was prepared in the same manner, but no sulfite was added. A colorimeter (a device that measures how much light of a selected wavelength is absorbed by a sample) was used to measure the absorbance of each solution. The absorbances were corrected by subtracting the absorbance of the blank solution from each reading (see Table 1 and Figure 1).Experiment 2A 100 g fruit sample was ground in a food processor with 50 mL of H2O and the mixture was filtered. The food processor and remaining fruit were then washed with H2O, these washings were filtered, and the liquid was added to the sample solution. The coloring agent was added and the solution was diluted to 100 mL. The procedure was repeated for several fruits, and the absorbances were measured (see Table 2).Q.If Experiments 1 and 2 were repeated using a different coloring agent that produces a different color when it binds with sulfite, which of the following changes in procedure would be necessary?a)The new coloring agent should be added to the blank solution, but not to the sample solutions.b)Both of the coloring agents should be added to the blank solution and to all of the samples.c)The absorbance of the blank solution made with the new coloring agent should be added to the measured absorbances.d)The colorimeter should be set to measure at a different wavelength of light.Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions:Read the passages and choose the best answer to each question.PassageCertain preservatives known as sulfites are often added to fruit products to keep the fruit fresher longer. Use of sulfites is controversial because studies have linked sulfites to severe reactions in some asthmatics. Students performed 2 experiments to measure sulfite levels.Experiment 1Four solutions, each containing a different amount of sulfite dissolved in H2 O were prepared. A coloring agent was added that binds with sulfite to form a red compound that strongly absorbs light of a specific wavelength, and each solution was diluted to 100 mL. A blank solution was prepared in the same manner, but no sulfite was added. A colorimeter (a device that measures how much light of a selected wavelength is absorbed by a sample) was used to measure the absorbance of each solution. The absorbances were corrected by subtracting the absorbance of the blank solution from each reading (see Table 1 and Figure 1).Experiment 2A 100 g fruit sample was ground in a food processor with 50 mL of H2O and the mixture was filtered. The food processor and remaining fruit were then washed with H2O, these washings were filtered, and the liquid was added to the sample solution. The coloring agent was added and the solution was diluted to 100 mL. The procedure was repeated for several fruits, and the absorbances were measured (see Table 2).Q.If Experiments 1 and 2 were repeated using a different coloring agent that produces a different color when it binds with sulfite, which of the following changes in procedure would be necessary?a)The new coloring agent should be added to the blank solution, but not to the sample solutions.b)Both of the coloring agents should be added to the blank solution and to all of the samples.c)The absorbance of the blank solution made with the new coloring agent should be added to the measured absorbances.d)The colorimeter should be set to measure at a different wavelength of light.Correct answer is option 'D'. Can you explain this answer?.
Solutions for Directions:Read the passages and choose the best answer to each question.PassageCertain preservatives known as sulfites are often added to fruit products to keep the fruit fresher longer. Use of sulfites is controversial because studies have linked sulfites to severe reactions in some asthmatics. Students performed 2 experiments to measure sulfite levels.Experiment 1Four solutions, each containing a different amount of sulfite dissolved in H2 O were prepared. A coloring agent was added that binds with sulfite to form a red compound that strongly absorbs light of a specific wavelength, and each solution was diluted to 100 mL. A blank solution was prepared in the same manner, but no sulfite was added. A colorimeter (a device that measures how much light of a selected wavelength is absorbed by a sample) was used to measure the absorbance of each solution. The absorbances were corrected by subtracting the absorbance of the blank solution from each reading (see Table 1 and Figure 1).Experiment 2A 100 g fruit sample was ground in a food processor with 50 mL of H2O and the mixture was filtered. The food processor and remaining fruit were then washed with H2O, these washings were filtered, and the liquid was added to the sample solution. The coloring agent was added and the solution was diluted to 100 mL. The procedure was repeated for several fruits, and the absorbances were measured (see Table 2).Q.If Experiments 1 and 2 were repeated using a different coloring agent that produces a different color when it binds with sulfite, which of the following changes in procedure would be necessary?a)The new coloring agent should be added to the blank solution, but not to the sample solutions.b)Both of the coloring agents should be added to the blank solution and to all of the samples.c)The absorbance of the blank solution made with the new coloring agent should be added to the measured absorbances.d)The colorimeter should be set to measure at a different wavelength of light.Correct answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for ACT.
Download more important topics, notes, lectures and mock test series for ACT Exam by signing up for free.
Here you can find the meaning of Directions:Read the passages and choose the best answer to each question.PassageCertain preservatives known as sulfites are often added to fruit products to keep the fruit fresher longer. Use of sulfites is controversial because studies have linked sulfites to severe reactions in some asthmatics. Students performed 2 experiments to measure sulfite levels.Experiment 1Four solutions, each containing a different amount of sulfite dissolved in H2 O were prepared. A coloring agent was added that binds with sulfite to form a red compound that strongly absorbs light of a specific wavelength, and each solution was diluted to 100 mL. A blank solution was prepared in the same manner, but no sulfite was added. A colorimeter (a device that measures how much light of a selected wavelength is absorbed by a sample) was used to measure the absorbance of each solution. The absorbances were corrected by subtracting the absorbance of the blank solution from each reading (see Table 1 and Figure 1).Experiment 2A 100 g fruit sample was ground in a food processor with 50 mL of H2O and the mixture was filtered. The food processor and remaining fruit were then washed with H2O, these washings were filtered, and the liquid was added to the sample solution. The coloring agent was added and the solution was diluted to 100 mL. The procedure was repeated for several fruits, and the absorbances were measured (see Table 2).Q.If Experiments 1 and 2 were repeated using a different coloring agent that produces a different color when it binds with sulfite, which of the following changes in procedure would be necessary?a)The new coloring agent should be added to the blank solution, but not to the sample solutions.b)Both of the coloring agents should be added to the blank solution and to all of the samples.c)The absorbance of the blank solution made with the new coloring agent should be added to the measured absorbances.d)The colorimeter should be set to measure at a different wavelength of light.Correct answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Directions:Read the passages and choose the best answer to each question.PassageCertain preservatives known as sulfites are often added to fruit products to keep the fruit fresher longer. Use of sulfites is controversial because studies have linked sulfites to severe reactions in some asthmatics. Students performed 2 experiments to measure sulfite levels.Experiment 1Four solutions, each containing a different amount of sulfite dissolved in H2 O were prepared. A coloring agent was added that binds with sulfite to form a red compound that strongly absorbs light of a specific wavelength, and each solution was diluted to 100 mL. A blank solution was prepared in the same manner, but no sulfite was added. A colorimeter (a device that measures how much light of a selected wavelength is absorbed by a sample) was used to measure the absorbance of each solution. The absorbances were corrected by subtracting the absorbance of the blank solution from each reading (see Table 1 and Figure 1).Experiment 2A 100 g fruit sample was ground in a food processor with 50 mL of H2O and the mixture was filtered. The food processor and remaining fruit were then washed with H2O, these washings were filtered, and the liquid was added to the sample solution. The coloring agent was added and the solution was diluted to 100 mL. The procedure was repeated for several fruits, and the absorbances were measured (see Table 2).Q.If Experiments 1 and 2 were repeated using a different coloring agent that produces a different color when it binds with sulfite, which of the following changes in procedure would be necessary?a)The new coloring agent should be added to the blank solution, but not to the sample solutions.b)Both of the coloring agents should be added to the blank solution and to all of the samples.c)The absorbance of the blank solution made with the new coloring agent should be added to the measured absorbances.d)The colorimeter should be set to measure at a different wavelength of light.Correct answer is option 'D'. Can you explain this answer?, a detailed solution for Directions:Read the passages and choose the best answer to each question.PassageCertain preservatives known as sulfites are often added to fruit products to keep the fruit fresher longer. Use of sulfites is controversial because studies have linked sulfites to severe reactions in some asthmatics. Students performed 2 experiments to measure sulfite levels.Experiment 1Four solutions, each containing a different amount of sulfite dissolved in H2 O were prepared. A coloring agent was added that binds with sulfite to form a red compound that strongly absorbs light of a specific wavelength, and each solution was diluted to 100 mL. A blank solution was prepared in the same manner, but no sulfite was added. A colorimeter (a device that measures how much light of a selected wavelength is absorbed by a sample) was used to measure the absorbance of each solution. The absorbances were corrected by subtracting the absorbance of the blank solution from each reading (see Table 1 and Figure 1).Experiment 2A 100 g fruit sample was ground in a food processor with 50 mL of H2O and the mixture was filtered. The food processor and remaining fruit were then washed with H2O, these washings were filtered, and the liquid was added to the sample solution. The coloring agent was added and the solution was diluted to 100 mL. The procedure was repeated for several fruits, and the absorbances were measured (see Table 2).Q.If Experiments 1 and 2 were repeated using a different coloring agent that produces a different color when it binds with sulfite, which of the following changes in procedure would be necessary?a)The new coloring agent should be added to the blank solution, but not to the sample solutions.b)Both of the coloring agents should be added to the blank solution and to all of the samples.c)The absorbance of the blank solution made with the new coloring agent should be added to the measured absorbances.d)The colorimeter should be set to measure at a different wavelength of light.Correct answer is option 'D'. Can you explain this answer? has been provided alongside types of Directions:Read the passages and choose the best answer to each question.PassageCertain preservatives known as sulfites are often added to fruit products to keep the fruit fresher longer. Use of sulfites is controversial because studies have linked sulfites to severe reactions in some asthmatics. Students performed 2 experiments to measure sulfite levels.Experiment 1Four solutions, each containing a different amount of sulfite dissolved in H2 O were prepared. A coloring agent was added that binds with sulfite to form a red compound that strongly absorbs light of a specific wavelength, and each solution was diluted to 100 mL. A blank solution was prepared in the same manner, but no sulfite was added. A colorimeter (a device that measures how much light of a selected wavelength is absorbed by a sample) was used to measure the absorbance of each solution. The absorbances were corrected by subtracting the absorbance of the blank solution from each reading (see Table 1 and Figure 1).Experiment 2A 100 g fruit sample was ground in a food processor with 50 mL of H2O and the mixture was filtered. The food processor and remaining fruit were then washed with H2O, these washings were filtered, and the liquid was added to the sample solution. The coloring agent was added and the solution was diluted to 100 mL. The procedure was repeated for several fruits, and the absorbances were measured (see Table 2).Q.If Experiments 1 and 2 were repeated using a different coloring agent that produces a different color when it binds with sulfite, which of the following changes in procedure would be necessary?a)The new coloring agent should be added to the blank solution, but not to the sample solutions.b)Both of the coloring agents should be added to the blank solution and to all of the samples.c)The absorbance of the blank solution made with the new coloring agent should be added to the measured absorbances.d)The colorimeter should be set to measure at a different wavelength of light.Correct answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Directions:Read the passages and choose the best answer to each question.PassageCertain preservatives known as sulfites are often added to fruit products to keep the fruit fresher longer. Use of sulfites is controversial because studies have linked sulfites to severe reactions in some asthmatics. Students performed 2 experiments to measure sulfite levels.Experiment 1Four solutions, each containing a different amount of sulfite dissolved in H2 O were prepared. A coloring agent was added that binds with sulfite to form a red compound that strongly absorbs light of a specific wavelength, and each solution was diluted to 100 mL. A blank solution was prepared in the same manner, but no sulfite was added. A colorimeter (a device that measures how much light of a selected wavelength is absorbed by a sample) was used to measure the absorbance of each solution. The absorbances were corrected by subtracting the absorbance of the blank solution from each reading (see Table 1 and Figure 1).Experiment 2A 100 g fruit sample was ground in a food processor with 50 mL of H2O and the mixture was filtered. The food processor and remaining fruit were then washed with H2O, these washings were filtered, and the liquid was added to the sample solution. The coloring agent was added and the solution was diluted to 100 mL. The procedure was repeated for several fruits, and the absorbances were measured (see Table 2).Q.If Experiments 1 and 2 were repeated using a different coloring agent that produces a different color when it binds with sulfite, which of the following changes in procedure would be necessary?a)The new coloring agent should be added to the blank solution, but not to the sample solutions.b)Both of the coloring agents should be added to the blank solution and to all of the samples.c)The absorbance of the blank solution made with the new coloring agent should be added to the measured absorbances.d)The colorimeter should be set to measure at a different wavelength of light.Correct answer is option 'D'. Can you explain this answer? tests, examples and also practice ACT tests.

|
Explore Courses for ACT exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























