ACT Exam > ACT Questions > Directions:Read the passages and choose the b...
Start Learning for Free
Directions: Read the passages and choose the best answer to each question.
Passage
The molar heat of fusion is the amount of heat necessary to melt (or freeze) 1.00 mole of a substance at its melting point at a constant pressure. The molar heat of fusion for water is 6.02 kilojoules per mole (kJ/mol).
The equation for molar heat of fusion is:
q = ΔHfus(mass/molar mass)
In this equation, q is the total amount of heat involved, ΔHfus represents the molar heat of fusion (this value is a constant for a given substance), and (mass/molar mass) represents the number of moles of a given substance.
The following table lists molar heats of fusion, boiling points, and melting points for several elements.
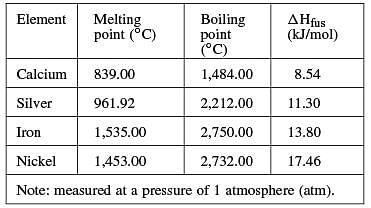
The molar heat of fusion is the amount of heat necessary to melt (or freeze) 1.00 mole of a substance at its melting point at a constant pressure. The molar heat of fusion for water is 6.02 kilojoules per mole (kJ/mol).
The equation for molar heat of fusion is:
q = ΔHfus(mass/molar mass)
In this equation, q is the total amount of heat involved, ΔHfus represents the molar heat of fusion (this value is a constant for a given substance), and (mass/molar mass) represents the number of moles of a given substance.
The following table lists molar heats of fusion, boiling points, and melting points for several elements.

Q. Molar heat of fusion is directly related to the strength of the forces that hold molecules together; strong forces make it difficult for molecules to break away into the liquid or gaseous phase. Data in the table support the conclusion that those forces are stronger in:
- a)calcium than in silver.
- b)silver than in nickel.
- c)iron than in calcium.
- d)iron than in nickel.
Correct answer is option 'C'. Can you explain this answer?
Most Upvoted Answer
Directions:Read the passages and choose the best answer to each questi...
You are given that the molar heat of fusion is directly related to the strength of the forces that hold molecules together; therefore, higher molar heats of fusion will indicate stronger bonds between molecules. Iron has a higher molar heat of fusion than calcium, so the forces holding molecules together will be stronger in iron than in calcium.

|
Explore Courses for ACT exam
|

|
Similar ACT Doubts
Directions:Read the passages and choose the best answer to each question.PassageThe molar heat of fusion is the amount of heat necessary to melt (or freeze) 1.00 mole of a substance at its melting point at a constant pressure. The molar heat of fusion for water is 6.02 kilojoules per mole (kJ/mol).The equation for molar heat of fusion is:q = ΔHfus(mass/molar mass)In this equation, q is the total amount of heat involved, ΔHfus represents the molar heat of fusion (this value is a constant for a given substance), and (mass/molar mass) represents the number of moles of a given substance.The following table lists molar heats of fusion, boiling points, and melting points for several elements.Q.Molar heat of fusion is directly related to the strength of the forces that hold molecules together; strong forces make it difficult for molecules to break away into the liquid or gaseous phase. Data in the table support the conclusion that those forces are stronger in:a)calcium than in silver.b)silver than in nickel.c)iron than in calcium.d)iron than in nickel.Correct answer is option 'C'. Can you explain this answer?
Question Description
Directions:Read the passages and choose the best answer to each question.PassageThe molar heat of fusion is the amount of heat necessary to melt (or freeze) 1.00 mole of a substance at its melting point at a constant pressure. The molar heat of fusion for water is 6.02 kilojoules per mole (kJ/mol).The equation for molar heat of fusion is:q = ΔHfus(mass/molar mass)In this equation, q is the total amount of heat involved, ΔHfus represents the molar heat of fusion (this value is a constant for a given substance), and (mass/molar mass) represents the number of moles of a given substance.The following table lists molar heats of fusion, boiling points, and melting points for several elements.Q.Molar heat of fusion is directly related to the strength of the forces that hold molecules together; strong forces make it difficult for molecules to break away into the liquid or gaseous phase. Data in the table support the conclusion that those forces are stronger in:a)calcium than in silver.b)silver than in nickel.c)iron than in calcium.d)iron than in nickel.Correct answer is option 'C'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Directions:Read the passages and choose the best answer to each question.PassageThe molar heat of fusion is the amount of heat necessary to melt (or freeze) 1.00 mole of a substance at its melting point at a constant pressure. The molar heat of fusion for water is 6.02 kilojoules per mole (kJ/mol).The equation for molar heat of fusion is:q = ΔHfus(mass/molar mass)In this equation, q is the total amount of heat involved, ΔHfus represents the molar heat of fusion (this value is a constant for a given substance), and (mass/molar mass) represents the number of moles of a given substance.The following table lists molar heats of fusion, boiling points, and melting points for several elements.Q.Molar heat of fusion is directly related to the strength of the forces that hold molecules together; strong forces make it difficult for molecules to break away into the liquid or gaseous phase. Data in the table support the conclusion that those forces are stronger in:a)calcium than in silver.b)silver than in nickel.c)iron than in calcium.d)iron than in nickel.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions:Read the passages and choose the best answer to each question.PassageThe molar heat of fusion is the amount of heat necessary to melt (or freeze) 1.00 mole of a substance at its melting point at a constant pressure. The molar heat of fusion for water is 6.02 kilojoules per mole (kJ/mol).The equation for molar heat of fusion is:q = ΔHfus(mass/molar mass)In this equation, q is the total amount of heat involved, ΔHfus represents the molar heat of fusion (this value is a constant for a given substance), and (mass/molar mass) represents the number of moles of a given substance.The following table lists molar heats of fusion, boiling points, and melting points for several elements.Q.Molar heat of fusion is directly related to the strength of the forces that hold molecules together; strong forces make it difficult for molecules to break away into the liquid or gaseous phase. Data in the table support the conclusion that those forces are stronger in:a)calcium than in silver.b)silver than in nickel.c)iron than in calcium.d)iron than in nickel.Correct answer is option 'C'. Can you explain this answer?.
Directions:Read the passages and choose the best answer to each question.PassageThe molar heat of fusion is the amount of heat necessary to melt (or freeze) 1.00 mole of a substance at its melting point at a constant pressure. The molar heat of fusion for water is 6.02 kilojoules per mole (kJ/mol).The equation for molar heat of fusion is:q = ΔHfus(mass/molar mass)In this equation, q is the total amount of heat involved, ΔHfus represents the molar heat of fusion (this value is a constant for a given substance), and (mass/molar mass) represents the number of moles of a given substance.The following table lists molar heats of fusion, boiling points, and melting points for several elements.Q.Molar heat of fusion is directly related to the strength of the forces that hold molecules together; strong forces make it difficult for molecules to break away into the liquid or gaseous phase. Data in the table support the conclusion that those forces are stronger in:a)calcium than in silver.b)silver than in nickel.c)iron than in calcium.d)iron than in nickel.Correct answer is option 'C'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Directions:Read the passages and choose the best answer to each question.PassageThe molar heat of fusion is the amount of heat necessary to melt (or freeze) 1.00 mole of a substance at its melting point at a constant pressure. The molar heat of fusion for water is 6.02 kilojoules per mole (kJ/mol).The equation for molar heat of fusion is:q = ΔHfus(mass/molar mass)In this equation, q is the total amount of heat involved, ΔHfus represents the molar heat of fusion (this value is a constant for a given substance), and (mass/molar mass) represents the number of moles of a given substance.The following table lists molar heats of fusion, boiling points, and melting points for several elements.Q.Molar heat of fusion is directly related to the strength of the forces that hold molecules together; strong forces make it difficult for molecules to break away into the liquid or gaseous phase. Data in the table support the conclusion that those forces are stronger in:a)calcium than in silver.b)silver than in nickel.c)iron than in calcium.d)iron than in nickel.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions:Read the passages and choose the best answer to each question.PassageThe molar heat of fusion is the amount of heat necessary to melt (or freeze) 1.00 mole of a substance at its melting point at a constant pressure. The molar heat of fusion for water is 6.02 kilojoules per mole (kJ/mol).The equation for molar heat of fusion is:q = ΔHfus(mass/molar mass)In this equation, q is the total amount of heat involved, ΔHfus represents the molar heat of fusion (this value is a constant for a given substance), and (mass/molar mass) represents the number of moles of a given substance.The following table lists molar heats of fusion, boiling points, and melting points for several elements.Q.Molar heat of fusion is directly related to the strength of the forces that hold molecules together; strong forces make it difficult for molecules to break away into the liquid or gaseous phase. Data in the table support the conclusion that those forces are stronger in:a)calcium than in silver.b)silver than in nickel.c)iron than in calcium.d)iron than in nickel.Correct answer is option 'C'. Can you explain this answer?.
Solutions for Directions:Read the passages and choose the best answer to each question.PassageThe molar heat of fusion is the amount of heat necessary to melt (or freeze) 1.00 mole of a substance at its melting point at a constant pressure. The molar heat of fusion for water is 6.02 kilojoules per mole (kJ/mol).The equation for molar heat of fusion is:q = ΔHfus(mass/molar mass)In this equation, q is the total amount of heat involved, ΔHfus represents the molar heat of fusion (this value is a constant for a given substance), and (mass/molar mass) represents the number of moles of a given substance.The following table lists molar heats of fusion, boiling points, and melting points for several elements.Q.Molar heat of fusion is directly related to the strength of the forces that hold molecules together; strong forces make it difficult for molecules to break away into the liquid or gaseous phase. Data in the table support the conclusion that those forces are stronger in:a)calcium than in silver.b)silver than in nickel.c)iron than in calcium.d)iron than in nickel.Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for ACT.
Download more important topics, notes, lectures and mock test series for ACT Exam by signing up for free.
Here you can find the meaning of Directions:Read the passages and choose the best answer to each question.PassageThe molar heat of fusion is the amount of heat necessary to melt (or freeze) 1.00 mole of a substance at its melting point at a constant pressure. The molar heat of fusion for water is 6.02 kilojoules per mole (kJ/mol).The equation for molar heat of fusion is:q = ΔHfus(mass/molar mass)In this equation, q is the total amount of heat involved, ΔHfus represents the molar heat of fusion (this value is a constant for a given substance), and (mass/molar mass) represents the number of moles of a given substance.The following table lists molar heats of fusion, boiling points, and melting points for several elements.Q.Molar heat of fusion is directly related to the strength of the forces that hold molecules together; strong forces make it difficult for molecules to break away into the liquid or gaseous phase. Data in the table support the conclusion that those forces are stronger in:a)calcium than in silver.b)silver than in nickel.c)iron than in calcium.d)iron than in nickel.Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Directions:Read the passages and choose the best answer to each question.PassageThe molar heat of fusion is the amount of heat necessary to melt (or freeze) 1.00 mole of a substance at its melting point at a constant pressure. The molar heat of fusion for water is 6.02 kilojoules per mole (kJ/mol).The equation for molar heat of fusion is:q = ΔHfus(mass/molar mass)In this equation, q is the total amount of heat involved, ΔHfus represents the molar heat of fusion (this value is a constant for a given substance), and (mass/molar mass) represents the number of moles of a given substance.The following table lists molar heats of fusion, boiling points, and melting points for several elements.Q.Molar heat of fusion is directly related to the strength of the forces that hold molecules together; strong forces make it difficult for molecules to break away into the liquid or gaseous phase. Data in the table support the conclusion that those forces are stronger in:a)calcium than in silver.b)silver than in nickel.c)iron than in calcium.d)iron than in nickel.Correct answer is option 'C'. Can you explain this answer?, a detailed solution for Directions:Read the passages and choose the best answer to each question.PassageThe molar heat of fusion is the amount of heat necessary to melt (or freeze) 1.00 mole of a substance at its melting point at a constant pressure. The molar heat of fusion for water is 6.02 kilojoules per mole (kJ/mol).The equation for molar heat of fusion is:q = ΔHfus(mass/molar mass)In this equation, q is the total amount of heat involved, ΔHfus represents the molar heat of fusion (this value is a constant for a given substance), and (mass/molar mass) represents the number of moles of a given substance.The following table lists molar heats of fusion, boiling points, and melting points for several elements.Q.Molar heat of fusion is directly related to the strength of the forces that hold molecules together; strong forces make it difficult for molecules to break away into the liquid or gaseous phase. Data in the table support the conclusion that those forces are stronger in:a)calcium than in silver.b)silver than in nickel.c)iron than in calcium.d)iron than in nickel.Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of Directions:Read the passages and choose the best answer to each question.PassageThe molar heat of fusion is the amount of heat necessary to melt (or freeze) 1.00 mole of a substance at its melting point at a constant pressure. The molar heat of fusion for water is 6.02 kilojoules per mole (kJ/mol).The equation for molar heat of fusion is:q = ΔHfus(mass/molar mass)In this equation, q is the total amount of heat involved, ΔHfus represents the molar heat of fusion (this value is a constant for a given substance), and (mass/molar mass) represents the number of moles of a given substance.The following table lists molar heats of fusion, boiling points, and melting points for several elements.Q.Molar heat of fusion is directly related to the strength of the forces that hold molecules together; strong forces make it difficult for molecules to break away into the liquid or gaseous phase. Data in the table support the conclusion that those forces are stronger in:a)calcium than in silver.b)silver than in nickel.c)iron than in calcium.d)iron than in nickel.Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Directions:Read the passages and choose the best answer to each question.PassageThe molar heat of fusion is the amount of heat necessary to melt (or freeze) 1.00 mole of a substance at its melting point at a constant pressure. The molar heat of fusion for water is 6.02 kilojoules per mole (kJ/mol).The equation for molar heat of fusion is:q = ΔHfus(mass/molar mass)In this equation, q is the total amount of heat involved, ΔHfus represents the molar heat of fusion (this value is a constant for a given substance), and (mass/molar mass) represents the number of moles of a given substance.The following table lists molar heats of fusion, boiling points, and melting points for several elements.Q.Molar heat of fusion is directly related to the strength of the forces that hold molecules together; strong forces make it difficult for molecules to break away into the liquid or gaseous phase. Data in the table support the conclusion that those forces are stronger in:a)calcium than in silver.b)silver than in nickel.c)iron than in calcium.d)iron than in nickel.Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice ACT tests.

|
Explore Courses for ACT exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























