MCAT Exam > MCAT Questions > Which of the following statements can reasona...
Start Learning for Free
Which of the following statements can reasonably be deduced from the phase diagram of helium below?
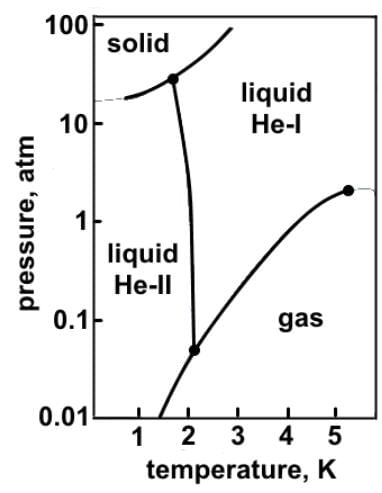

- a)At atmospheric pressure, helium can exist in all three phases, as well as a supercritical fluid phase near absolute zero.
- b)Solid and gaseous helium never exist in equilibrium with each other at any temperature or pressure.
- c)Helium does not exist as a solid below 25 atmospheres due to its low mass.
- d)At room temperature, helium exists as a gas, and as pressure increases eventually becomes a solid.
Correct answer is option 'B'. Can you explain this answer?
Verified Answer
Which of the following statements can reasonably be deduced from the p...
Helium has some peculiar features in its phase diagram. A typical phase diagram looks like the following:
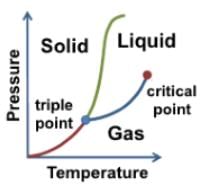
At room temperature, helium exists as a gas, and as pressure increases it eventually becomes a liquid. Looking at the line boundary between solid and the two liquid phases, the line curves up. Since the graph is using the logarithmic scale, the line curves more steeply than it would seem.
At atmospheric pressure, helium can exist as a supercritical fluid (He-II), normal liquid (He-I), and gas, but not as a solid.
Helium does not exist as a solid below 25 atmospheres due to the weak London dispersion forces that exist between noble gas atoms.
Most Upvoted Answer
Which of the following statements can reasonably be deduced from the p...
Helium has some peculiar features in its phase diagram. A typical phase diagram looks like the following:

At room temperature, helium exists as a gas, and as pressure increases it eventually becomes a liquid. Looking at the line boundary between solid and the two liquid phases, the line curves up. Since the graph is using the logarithmic scale, the line curves more steeply than it would seem.
At atmospheric pressure, helium can exist as a supercritical fluid (He-II), normal liquid (He-I), and gas, but not as a solid.
Helium does not exist as a solid below 25 atmospheres due to the weak London dispersion forces that exist between noble gas atoms.

|
Explore Courses for MCAT exam
|

|
Question Description
Which of the following statements can reasonably be deduced from the phase diagram of helium below?a)At atmospheric pressure, helium can exist in all three phases, as well as a supercritical fluid phase near absolute zero.b)Solid and gaseous helium never exist in equilibrium with each other at any temperature or pressure.c)Helium does not exist as a solid below 25atmospheres due to its low mass.d)At room temperature, helium exists as a gas, and as pressure increases eventually becomes a solid.Correct answer is option 'B'. Can you explain this answer? for MCAT 2025 is part of MCAT preparation. The Question and answers have been prepared according to the MCAT exam syllabus. Information about Which of the following statements can reasonably be deduced from the phase diagram of helium below?a)At atmospheric pressure, helium can exist in all three phases, as well as a supercritical fluid phase near absolute zero.b)Solid and gaseous helium never exist in equilibrium with each other at any temperature or pressure.c)Helium does not exist as a solid below 25atmospheres due to its low mass.d)At room temperature, helium exists as a gas, and as pressure increases eventually becomes a solid.Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for MCAT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following statements can reasonably be deduced from the phase diagram of helium below?a)At atmospheric pressure, helium can exist in all three phases, as well as a supercritical fluid phase near absolute zero.b)Solid and gaseous helium never exist in equilibrium with each other at any temperature or pressure.c)Helium does not exist as a solid below 25atmospheres due to its low mass.d)At room temperature, helium exists as a gas, and as pressure increases eventually becomes a solid.Correct answer is option 'B'. Can you explain this answer?.
Which of the following statements can reasonably be deduced from the phase diagram of helium below?a)At atmospheric pressure, helium can exist in all three phases, as well as a supercritical fluid phase near absolute zero.b)Solid and gaseous helium never exist in equilibrium with each other at any temperature or pressure.c)Helium does not exist as a solid below 25atmospheres due to its low mass.d)At room temperature, helium exists as a gas, and as pressure increases eventually becomes a solid.Correct answer is option 'B'. Can you explain this answer? for MCAT 2025 is part of MCAT preparation. The Question and answers have been prepared according to the MCAT exam syllabus. Information about Which of the following statements can reasonably be deduced from the phase diagram of helium below?a)At atmospheric pressure, helium can exist in all three phases, as well as a supercritical fluid phase near absolute zero.b)Solid and gaseous helium never exist in equilibrium with each other at any temperature or pressure.c)Helium does not exist as a solid below 25atmospheres due to its low mass.d)At room temperature, helium exists as a gas, and as pressure increases eventually becomes a solid.Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for MCAT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following statements can reasonably be deduced from the phase diagram of helium below?a)At atmospheric pressure, helium can exist in all three phases, as well as a supercritical fluid phase near absolute zero.b)Solid and gaseous helium never exist in equilibrium with each other at any temperature or pressure.c)Helium does not exist as a solid below 25atmospheres due to its low mass.d)At room temperature, helium exists as a gas, and as pressure increases eventually becomes a solid.Correct answer is option 'B'. Can you explain this answer?.
Solutions for Which of the following statements can reasonably be deduced from the phase diagram of helium below?a)At atmospheric pressure, helium can exist in all three phases, as well as a supercritical fluid phase near absolute zero.b)Solid and gaseous helium never exist in equilibrium with each other at any temperature or pressure.c)Helium does not exist as a solid below 25atmospheres due to its low mass.d)At room temperature, helium exists as a gas, and as pressure increases eventually becomes a solid.Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for MCAT.
Download more important topics, notes, lectures and mock test series for MCAT Exam by signing up for free.
Here you can find the meaning of Which of the following statements can reasonably be deduced from the phase diagram of helium below?a)At atmospheric pressure, helium can exist in all three phases, as well as a supercritical fluid phase near absolute zero.b)Solid and gaseous helium never exist in equilibrium with each other at any temperature or pressure.c)Helium does not exist as a solid below 25atmospheres due to its low mass.d)At room temperature, helium exists as a gas, and as pressure increases eventually becomes a solid.Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following statements can reasonably be deduced from the phase diagram of helium below?a)At atmospheric pressure, helium can exist in all three phases, as well as a supercritical fluid phase near absolute zero.b)Solid and gaseous helium never exist in equilibrium with each other at any temperature or pressure.c)Helium does not exist as a solid below 25atmospheres due to its low mass.d)At room temperature, helium exists as a gas, and as pressure increases eventually becomes a solid.Correct answer is option 'B'. Can you explain this answer?, a detailed solution for Which of the following statements can reasonably be deduced from the phase diagram of helium below?a)At atmospheric pressure, helium can exist in all three phases, as well as a supercritical fluid phase near absolute zero.b)Solid and gaseous helium never exist in equilibrium with each other at any temperature or pressure.c)Helium does not exist as a solid below 25atmospheres due to its low mass.d)At room temperature, helium exists as a gas, and as pressure increases eventually becomes a solid.Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of Which of the following statements can reasonably be deduced from the phase diagram of helium below?a)At atmospheric pressure, helium can exist in all three phases, as well as a supercritical fluid phase near absolute zero.b)Solid and gaseous helium never exist in equilibrium with each other at any temperature or pressure.c)Helium does not exist as a solid below 25atmospheres due to its low mass.d)At room temperature, helium exists as a gas, and as pressure increases eventually becomes a solid.Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following statements can reasonably be deduced from the phase diagram of helium below?a)At atmospheric pressure, helium can exist in all three phases, as well as a supercritical fluid phase near absolute zero.b)Solid and gaseous helium never exist in equilibrium with each other at any temperature or pressure.c)Helium does not exist as a solid below 25atmospheres due to its low mass.d)At room temperature, helium exists as a gas, and as pressure increases eventually becomes a solid.Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice MCAT tests.

|
Explore Courses for MCAT exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















