ACT Exam > ACT Questions > Directions:Read the passages and choose the b...
Start Learning for Free
Directions: Read the passages and choose the best answer to each question.
Passage
Yeast is a unicellular fungus, and is arguably one of the most important members of the fungus family, primarily because of its involvement in the process of fermentation.
During this process, yeast breaks down sucrose into alcohol and carbon dioxide. The chemical equation for fermentation is given below. Scientists study how to induce fermentation in yeast most effectively.
C12H22O11 + H2O → 4C2H5OH + 4CO2
Experiment 1
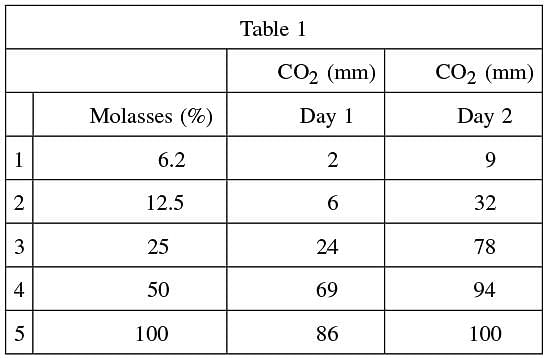
Since yeast needs sucrose to ferment and molasses is 60% sucrose, scientists first study yeast grown in molasses.
Five test tubes are filled with .4 grams of yeast and various molasses concentrations. Carbon dioxide levels are measured as an indication of fermentation for each test tube after one day and again after two days. These levels are shown in Table 1.
Experiment 2
Five different test tubes are filled with .4 grams of yeast and various pure sucrose dilutions. Carbon dioxide levels are then measured as an indication of fermentation after one and two days. These levels are shown in Table 2.

Experiment 3
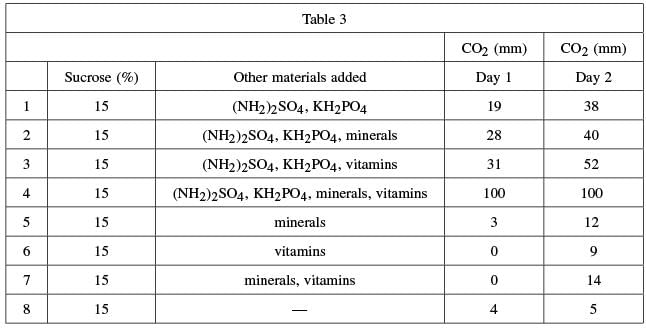
Eight more test tubes were filled with .4 grams of yeast and a 15% sucrose solution. Various combinations of ammonium sulfate, potassium dihydrogen phosphate, minerals, and vitamins were added to the test tubes. Carbon dioxide levels were measured after one and two days. These levels are shown in Table 3.
Passage
Yeast is a unicellular fungus, and is arguably one of the most important members of the fungus family, primarily because of its involvement in the process of fermentation.
During this process, yeast breaks down sucrose into alcohol and carbon dioxide. The chemical equation for fermentation is given below. Scientists study how to induce fermentation in yeast most effectively.
C12H22O11 + H2O → 4C2H5OH + 4CO2
Experiment 1
Since yeast needs sucrose to ferment and molasses is 60% sucrose, scientists first study yeast grown in molasses.
Five test tubes are filled with .4 grams of yeast and various molasses concentrations. Carbon dioxide levels are measured as an indication of fermentation for each test tube after one day and again after two days. These levels are shown in Table 1.

Experiment 2
Five different test tubes are filled with .4 grams of yeast and various pure sucrose dilutions. Carbon dioxide levels are then measured as an indication of fermentation after one and two days. These levels are shown in Table 2.

Experiment 3
Eight more test tubes were filled with .4 grams of yeast and a 15% sucrose solution. Various combinations of ammonium sulfate, potassium dihydrogen phosphate, minerals, and vitamins were added to the test tubes. Carbon dioxide levels were measured after one and two days. These levels are shown in Table 3.

Q. Which of the following is a weakness in the design of Experiment 3?
- a)Different amounts of yeast were used in each test tube.
- b)Varying concentrations of sucrose were used.
- c)There was no control group in which the experimental treatment was not applied.
- d)All possible combinations of nutrients were not tested.
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
Directions:Read the passages and choose the best answer to each questi...
The best answer is D. The best way to answer this question is by the process of elimination.
Answer choice A is incorrect because 0.4 grams of yeast were added to each of the eight test tubes. Answer choice B is incorrect because a 15% sucrose solution was added to each test tube. Answer choice C is incorrect because the experimental treatment was not applied in test tube 8. Only answer choice D represents a weakness in the design of the experiment, as different nutrient combinations could yield different results.
Answer choice A is incorrect because 0.4 grams of yeast were added to each of the eight test tubes. Answer choice B is incorrect because a 15% sucrose solution was added to each test tube. Answer choice C is incorrect because the experimental treatment was not applied in test tube 8. Only answer choice D represents a weakness in the design of the experiment, as different nutrient combinations could yield different results.
Most Upvoted Answer
Directions:Read the passages and choose the best answer to each questi...
The best answer is D. The best way to answer this question is by the process of elimination.
Answer choice A is incorrect because 0.4 grams of yeast were added to each of the eight test tubes. Answer choice B is incorrect because a 15% sucrose solution was added to each test tube. Answer choice C is incorrect because the experimental treatment was not applied in test tube 8. Only answer choice D represents a weakness in the design of the experiment, as different nutrient combinations could yield different results.
Answer choice A is incorrect because 0.4 grams of yeast were added to each of the eight test tubes. Answer choice B is incorrect because a 15% sucrose solution was added to each test tube. Answer choice C is incorrect because the experimental treatment was not applied in test tube 8. Only answer choice D represents a weakness in the design of the experiment, as different nutrient combinations could yield different results.

|
Explore Courses for ACT exam
|

|
Directions:Read the passages and choose the best answer to each question.PassageYeast is a unicellular fungus, and is arguably one of the most important members of the fungus family, primarily because of its involvement in the process of fermentation.During this process, yeast breaks down sucrose into alcohol and carbon dioxide. The chemical equation for fermentation is given below. Scientists study how to induce fermentation in yeast most effectively.C12H22O11 + H2O → 4C2H5OH + 4CO2Experiment 1Since yeast needs sucrose to ferment and molasses is 60% sucrose, scientists first study yeast grown in molasses.Five test tubes are filled with .4 grams of yeast and various molasses concentrations. Carbon dioxide levels are measured as an indication of fermentation for each test tube after one day and again after two days. These levels are shown in Table 1.Experiment 2Five different test tubes are filled with .4 grams of yeast and various pure sucrose dilutions. Carbon dioxide levels are then measured as an indication of fermentation after one and two days. These levels are shown in Table 2.Experiment 3Eight more test tubes were filled with .4 grams of yeast and a 15% sucrose solution. Various combinations of ammonium sulfate, potassium dihydrogen phosphate, minerals, and vitamins were added to the test tubes. Carbon dioxide levels were measured after one and two days. These levels are shown in Table 3.Q.Which of the following is a weakness in the design of Experiment 3?a)Different amounts of yeast were used in each test tube.b)Varying concentrations of sucrose were used.c)There was no control group in which the experimental treatment was not applied.d)All possible combinations of nutrients were not tested.Correct answer is option 'D'. Can you explain this answer?
Question Description
Directions:Read the passages and choose the best answer to each question.PassageYeast is a unicellular fungus, and is arguably one of the most important members of the fungus family, primarily because of its involvement in the process of fermentation.During this process, yeast breaks down sucrose into alcohol and carbon dioxide. The chemical equation for fermentation is given below. Scientists study how to induce fermentation in yeast most effectively.C12H22O11 + H2O → 4C2H5OH + 4CO2Experiment 1Since yeast needs sucrose to ferment and molasses is 60% sucrose, scientists first study yeast grown in molasses.Five test tubes are filled with .4 grams of yeast and various molasses concentrations. Carbon dioxide levels are measured as an indication of fermentation for each test tube after one day and again after two days. These levels are shown in Table 1.Experiment 2Five different test tubes are filled with .4 grams of yeast and various pure sucrose dilutions. Carbon dioxide levels are then measured as an indication of fermentation after one and two days. These levels are shown in Table 2.Experiment 3Eight more test tubes were filled with .4 grams of yeast and a 15% sucrose solution. Various combinations of ammonium sulfate, potassium dihydrogen phosphate, minerals, and vitamins were added to the test tubes. Carbon dioxide levels were measured after one and two days. These levels are shown in Table 3.Q.Which of the following is a weakness in the design of Experiment 3?a)Different amounts of yeast were used in each test tube.b)Varying concentrations of sucrose were used.c)There was no control group in which the experimental treatment was not applied.d)All possible combinations of nutrients were not tested.Correct answer is option 'D'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Directions:Read the passages and choose the best answer to each question.PassageYeast is a unicellular fungus, and is arguably one of the most important members of the fungus family, primarily because of its involvement in the process of fermentation.During this process, yeast breaks down sucrose into alcohol and carbon dioxide. The chemical equation for fermentation is given below. Scientists study how to induce fermentation in yeast most effectively.C12H22O11 + H2O → 4C2H5OH + 4CO2Experiment 1Since yeast needs sucrose to ferment and molasses is 60% sucrose, scientists first study yeast grown in molasses.Five test tubes are filled with .4 grams of yeast and various molasses concentrations. Carbon dioxide levels are measured as an indication of fermentation for each test tube after one day and again after two days. These levels are shown in Table 1.Experiment 2Five different test tubes are filled with .4 grams of yeast and various pure sucrose dilutions. Carbon dioxide levels are then measured as an indication of fermentation after one and two days. These levels are shown in Table 2.Experiment 3Eight more test tubes were filled with .4 grams of yeast and a 15% sucrose solution. Various combinations of ammonium sulfate, potassium dihydrogen phosphate, minerals, and vitamins were added to the test tubes. Carbon dioxide levels were measured after one and two days. These levels are shown in Table 3.Q.Which of the following is a weakness in the design of Experiment 3?a)Different amounts of yeast were used in each test tube.b)Varying concentrations of sucrose were used.c)There was no control group in which the experimental treatment was not applied.d)All possible combinations of nutrients were not tested.Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions:Read the passages and choose the best answer to each question.PassageYeast is a unicellular fungus, and is arguably one of the most important members of the fungus family, primarily because of its involvement in the process of fermentation.During this process, yeast breaks down sucrose into alcohol and carbon dioxide. The chemical equation for fermentation is given below. Scientists study how to induce fermentation in yeast most effectively.C12H22O11 + H2O → 4C2H5OH + 4CO2Experiment 1Since yeast needs sucrose to ferment and molasses is 60% sucrose, scientists first study yeast grown in molasses.Five test tubes are filled with .4 grams of yeast and various molasses concentrations. Carbon dioxide levels are measured as an indication of fermentation for each test tube after one day and again after two days. These levels are shown in Table 1.Experiment 2Five different test tubes are filled with .4 grams of yeast and various pure sucrose dilutions. Carbon dioxide levels are then measured as an indication of fermentation after one and two days. These levels are shown in Table 2.Experiment 3Eight more test tubes were filled with .4 grams of yeast and a 15% sucrose solution. Various combinations of ammonium sulfate, potassium dihydrogen phosphate, minerals, and vitamins were added to the test tubes. Carbon dioxide levels were measured after one and two days. These levels are shown in Table 3.Q.Which of the following is a weakness in the design of Experiment 3?a)Different amounts of yeast were used in each test tube.b)Varying concentrations of sucrose were used.c)There was no control group in which the experimental treatment was not applied.d)All possible combinations of nutrients were not tested.Correct answer is option 'D'. Can you explain this answer?.
Directions:Read the passages and choose the best answer to each question.PassageYeast is a unicellular fungus, and is arguably one of the most important members of the fungus family, primarily because of its involvement in the process of fermentation.During this process, yeast breaks down sucrose into alcohol and carbon dioxide. The chemical equation for fermentation is given below. Scientists study how to induce fermentation in yeast most effectively.C12H22O11 + H2O → 4C2H5OH + 4CO2Experiment 1Since yeast needs sucrose to ferment and molasses is 60% sucrose, scientists first study yeast grown in molasses.Five test tubes are filled with .4 grams of yeast and various molasses concentrations. Carbon dioxide levels are measured as an indication of fermentation for each test tube after one day and again after two days. These levels are shown in Table 1.Experiment 2Five different test tubes are filled with .4 grams of yeast and various pure sucrose dilutions. Carbon dioxide levels are then measured as an indication of fermentation after one and two days. These levels are shown in Table 2.Experiment 3Eight more test tubes were filled with .4 grams of yeast and a 15% sucrose solution. Various combinations of ammonium sulfate, potassium dihydrogen phosphate, minerals, and vitamins were added to the test tubes. Carbon dioxide levels were measured after one and two days. These levels are shown in Table 3.Q.Which of the following is a weakness in the design of Experiment 3?a)Different amounts of yeast were used in each test tube.b)Varying concentrations of sucrose were used.c)There was no control group in which the experimental treatment was not applied.d)All possible combinations of nutrients were not tested.Correct answer is option 'D'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Directions:Read the passages and choose the best answer to each question.PassageYeast is a unicellular fungus, and is arguably one of the most important members of the fungus family, primarily because of its involvement in the process of fermentation.During this process, yeast breaks down sucrose into alcohol and carbon dioxide. The chemical equation for fermentation is given below. Scientists study how to induce fermentation in yeast most effectively.C12H22O11 + H2O → 4C2H5OH + 4CO2Experiment 1Since yeast needs sucrose to ferment and molasses is 60% sucrose, scientists first study yeast grown in molasses.Five test tubes are filled with .4 grams of yeast and various molasses concentrations. Carbon dioxide levels are measured as an indication of fermentation for each test tube after one day and again after two days. These levels are shown in Table 1.Experiment 2Five different test tubes are filled with .4 grams of yeast and various pure sucrose dilutions. Carbon dioxide levels are then measured as an indication of fermentation after one and two days. These levels are shown in Table 2.Experiment 3Eight more test tubes were filled with .4 grams of yeast and a 15% sucrose solution. Various combinations of ammonium sulfate, potassium dihydrogen phosphate, minerals, and vitamins were added to the test tubes. Carbon dioxide levels were measured after one and two days. These levels are shown in Table 3.Q.Which of the following is a weakness in the design of Experiment 3?a)Different amounts of yeast were used in each test tube.b)Varying concentrations of sucrose were used.c)There was no control group in which the experimental treatment was not applied.d)All possible combinations of nutrients were not tested.Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions:Read the passages and choose the best answer to each question.PassageYeast is a unicellular fungus, and is arguably one of the most important members of the fungus family, primarily because of its involvement in the process of fermentation.During this process, yeast breaks down sucrose into alcohol and carbon dioxide. The chemical equation for fermentation is given below. Scientists study how to induce fermentation in yeast most effectively.C12H22O11 + H2O → 4C2H5OH + 4CO2Experiment 1Since yeast needs sucrose to ferment and molasses is 60% sucrose, scientists first study yeast grown in molasses.Five test tubes are filled with .4 grams of yeast and various molasses concentrations. Carbon dioxide levels are measured as an indication of fermentation for each test tube after one day and again after two days. These levels are shown in Table 1.Experiment 2Five different test tubes are filled with .4 grams of yeast and various pure sucrose dilutions. Carbon dioxide levels are then measured as an indication of fermentation after one and two days. These levels are shown in Table 2.Experiment 3Eight more test tubes were filled with .4 grams of yeast and a 15% sucrose solution. Various combinations of ammonium sulfate, potassium dihydrogen phosphate, minerals, and vitamins were added to the test tubes. Carbon dioxide levels were measured after one and two days. These levels are shown in Table 3.Q.Which of the following is a weakness in the design of Experiment 3?a)Different amounts of yeast were used in each test tube.b)Varying concentrations of sucrose were used.c)There was no control group in which the experimental treatment was not applied.d)All possible combinations of nutrients were not tested.Correct answer is option 'D'. Can you explain this answer?.
Solutions for Directions:Read the passages and choose the best answer to each question.PassageYeast is a unicellular fungus, and is arguably one of the most important members of the fungus family, primarily because of its involvement in the process of fermentation.During this process, yeast breaks down sucrose into alcohol and carbon dioxide. The chemical equation for fermentation is given below. Scientists study how to induce fermentation in yeast most effectively.C12H22O11 + H2O → 4C2H5OH + 4CO2Experiment 1Since yeast needs sucrose to ferment and molasses is 60% sucrose, scientists first study yeast grown in molasses.Five test tubes are filled with .4 grams of yeast and various molasses concentrations. Carbon dioxide levels are measured as an indication of fermentation for each test tube after one day and again after two days. These levels are shown in Table 1.Experiment 2Five different test tubes are filled with .4 grams of yeast and various pure sucrose dilutions. Carbon dioxide levels are then measured as an indication of fermentation after one and two days. These levels are shown in Table 2.Experiment 3Eight more test tubes were filled with .4 grams of yeast and a 15% sucrose solution. Various combinations of ammonium sulfate, potassium dihydrogen phosphate, minerals, and vitamins were added to the test tubes. Carbon dioxide levels were measured after one and two days. These levels are shown in Table 3.Q.Which of the following is a weakness in the design of Experiment 3?a)Different amounts of yeast were used in each test tube.b)Varying concentrations of sucrose were used.c)There was no control group in which the experimental treatment was not applied.d)All possible combinations of nutrients were not tested.Correct answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for ACT.
Download more important topics, notes, lectures and mock test series for ACT Exam by signing up for free.
Here you can find the meaning of Directions:Read the passages and choose the best answer to each question.PassageYeast is a unicellular fungus, and is arguably one of the most important members of the fungus family, primarily because of its involvement in the process of fermentation.During this process, yeast breaks down sucrose into alcohol and carbon dioxide. The chemical equation for fermentation is given below. Scientists study how to induce fermentation in yeast most effectively.C12H22O11 + H2O → 4C2H5OH + 4CO2Experiment 1Since yeast needs sucrose to ferment and molasses is 60% sucrose, scientists first study yeast grown in molasses.Five test tubes are filled with .4 grams of yeast and various molasses concentrations. Carbon dioxide levels are measured as an indication of fermentation for each test tube after one day and again after two days. These levels are shown in Table 1.Experiment 2Five different test tubes are filled with .4 grams of yeast and various pure sucrose dilutions. Carbon dioxide levels are then measured as an indication of fermentation after one and two days. These levels are shown in Table 2.Experiment 3Eight more test tubes were filled with .4 grams of yeast and a 15% sucrose solution. Various combinations of ammonium sulfate, potassium dihydrogen phosphate, minerals, and vitamins were added to the test tubes. Carbon dioxide levels were measured after one and two days. These levels are shown in Table 3.Q.Which of the following is a weakness in the design of Experiment 3?a)Different amounts of yeast were used in each test tube.b)Varying concentrations of sucrose were used.c)There was no control group in which the experimental treatment was not applied.d)All possible combinations of nutrients were not tested.Correct answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Directions:Read the passages and choose the best answer to each question.PassageYeast is a unicellular fungus, and is arguably one of the most important members of the fungus family, primarily because of its involvement in the process of fermentation.During this process, yeast breaks down sucrose into alcohol and carbon dioxide. The chemical equation for fermentation is given below. Scientists study how to induce fermentation in yeast most effectively.C12H22O11 + H2O → 4C2H5OH + 4CO2Experiment 1Since yeast needs sucrose to ferment and molasses is 60% sucrose, scientists first study yeast grown in molasses.Five test tubes are filled with .4 grams of yeast and various molasses concentrations. Carbon dioxide levels are measured as an indication of fermentation for each test tube after one day and again after two days. These levels are shown in Table 1.Experiment 2Five different test tubes are filled with .4 grams of yeast and various pure sucrose dilutions. Carbon dioxide levels are then measured as an indication of fermentation after one and two days. These levels are shown in Table 2.Experiment 3Eight more test tubes were filled with .4 grams of yeast and a 15% sucrose solution. Various combinations of ammonium sulfate, potassium dihydrogen phosphate, minerals, and vitamins were added to the test tubes. Carbon dioxide levels were measured after one and two days. These levels are shown in Table 3.Q.Which of the following is a weakness in the design of Experiment 3?a)Different amounts of yeast were used in each test tube.b)Varying concentrations of sucrose were used.c)There was no control group in which the experimental treatment was not applied.d)All possible combinations of nutrients were not tested.Correct answer is option 'D'. Can you explain this answer?, a detailed solution for Directions:Read the passages and choose the best answer to each question.PassageYeast is a unicellular fungus, and is arguably one of the most important members of the fungus family, primarily because of its involvement in the process of fermentation.During this process, yeast breaks down sucrose into alcohol and carbon dioxide. The chemical equation for fermentation is given below. Scientists study how to induce fermentation in yeast most effectively.C12H22O11 + H2O → 4C2H5OH + 4CO2Experiment 1Since yeast needs sucrose to ferment and molasses is 60% sucrose, scientists first study yeast grown in molasses.Five test tubes are filled with .4 grams of yeast and various molasses concentrations. Carbon dioxide levels are measured as an indication of fermentation for each test tube after one day and again after two days. These levels are shown in Table 1.Experiment 2Five different test tubes are filled with .4 grams of yeast and various pure sucrose dilutions. Carbon dioxide levels are then measured as an indication of fermentation after one and two days. These levels are shown in Table 2.Experiment 3Eight more test tubes were filled with .4 grams of yeast and a 15% sucrose solution. Various combinations of ammonium sulfate, potassium dihydrogen phosphate, minerals, and vitamins were added to the test tubes. Carbon dioxide levels were measured after one and two days. These levels are shown in Table 3.Q.Which of the following is a weakness in the design of Experiment 3?a)Different amounts of yeast were used in each test tube.b)Varying concentrations of sucrose were used.c)There was no control group in which the experimental treatment was not applied.d)All possible combinations of nutrients were not tested.Correct answer is option 'D'. Can you explain this answer? has been provided alongside types of Directions:Read the passages and choose the best answer to each question.PassageYeast is a unicellular fungus, and is arguably one of the most important members of the fungus family, primarily because of its involvement in the process of fermentation.During this process, yeast breaks down sucrose into alcohol and carbon dioxide. The chemical equation for fermentation is given below. Scientists study how to induce fermentation in yeast most effectively.C12H22O11 + H2O → 4C2H5OH + 4CO2Experiment 1Since yeast needs sucrose to ferment and molasses is 60% sucrose, scientists first study yeast grown in molasses.Five test tubes are filled with .4 grams of yeast and various molasses concentrations. Carbon dioxide levels are measured as an indication of fermentation for each test tube after one day and again after two days. These levels are shown in Table 1.Experiment 2Five different test tubes are filled with .4 grams of yeast and various pure sucrose dilutions. Carbon dioxide levels are then measured as an indication of fermentation after one and two days. These levels are shown in Table 2.Experiment 3Eight more test tubes were filled with .4 grams of yeast and a 15% sucrose solution. Various combinations of ammonium sulfate, potassium dihydrogen phosphate, minerals, and vitamins were added to the test tubes. Carbon dioxide levels were measured after one and two days. These levels are shown in Table 3.Q.Which of the following is a weakness in the design of Experiment 3?a)Different amounts of yeast were used in each test tube.b)Varying concentrations of sucrose were used.c)There was no control group in which the experimental treatment was not applied.d)All possible combinations of nutrients were not tested.Correct answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Directions:Read the passages and choose the best answer to each question.PassageYeast is a unicellular fungus, and is arguably one of the most important members of the fungus family, primarily because of its involvement in the process of fermentation.During this process, yeast breaks down sucrose into alcohol and carbon dioxide. The chemical equation for fermentation is given below. Scientists study how to induce fermentation in yeast most effectively.C12H22O11 + H2O → 4C2H5OH + 4CO2Experiment 1Since yeast needs sucrose to ferment and molasses is 60% sucrose, scientists first study yeast grown in molasses.Five test tubes are filled with .4 grams of yeast and various molasses concentrations. Carbon dioxide levels are measured as an indication of fermentation for each test tube after one day and again after two days. These levels are shown in Table 1.Experiment 2Five different test tubes are filled with .4 grams of yeast and various pure sucrose dilutions. Carbon dioxide levels are then measured as an indication of fermentation after one and two days. These levels are shown in Table 2.Experiment 3Eight more test tubes were filled with .4 grams of yeast and a 15% sucrose solution. Various combinations of ammonium sulfate, potassium dihydrogen phosphate, minerals, and vitamins were added to the test tubes. Carbon dioxide levels were measured after one and two days. These levels are shown in Table 3.Q.Which of the following is a weakness in the design of Experiment 3?a)Different amounts of yeast were used in each test tube.b)Varying concentrations of sucrose were used.c)There was no control group in which the experimental treatment was not applied.d)All possible combinations of nutrients were not tested.Correct answer is option 'D'. Can you explain this answer? tests, examples and also practice ACT tests.

|
Explore Courses for ACT exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























