ACT Exam > ACT Questions > Directions:Read the passages and choose the b...
Start Learning for Free
Directions: Read the passages and choose the best answer to each question.
Passage
Students studied the effect of temperature on the conversion rates of two organic acids to their corresponding alcohols. The two organic acids studied were lactic acid (LA) and propionic acid (PA). Each acid was mixed with an Ru/C catalyst (to start the conversion) in an aqueous (water) solution. Lactic acid was found to break down into propylene glycol (PG), water, and various carbon side products. Propionic acid was found to break down into 1-propanol (1-PrOH), water, and various carbon side products. For all experiments, temperature was measured in degrees Kelvin (K).
Experiment 1
Students mixed an LA concentration of 0.5 moles (M) in a 50-milliliter (ml) aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from lactic acid to PG. The results are shown in Figure 1.
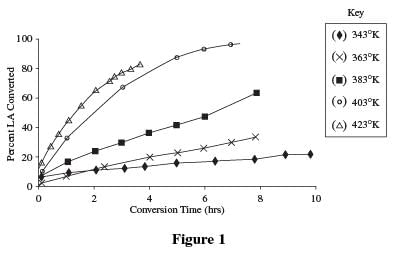
Experiment 2
Students mixed a PA concentration of 0.5 M in a 50 ml aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from propionic acid to 1-PrOH. The results are shown in Figure 2.
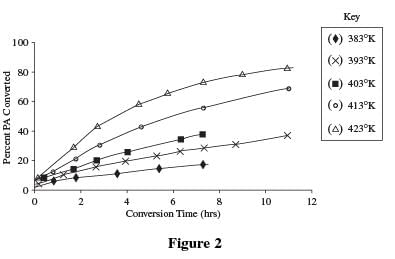
Passage
Students studied the effect of temperature on the conversion rates of two organic acids to their corresponding alcohols. The two organic acids studied were lactic acid (LA) and propionic acid (PA). Each acid was mixed with an Ru/C catalyst (to start the conversion) in an aqueous (water) solution. Lactic acid was found to break down into propylene glycol (PG), water, and various carbon side products. Propionic acid was found to break down into 1-propanol (1-PrOH), water, and various carbon side products. For all experiments, temperature was measured in degrees Kelvin (K).
Experiment 1
Students mixed an LA concentration of 0.5 moles (M) in a 50-milliliter (ml) aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from lactic acid to PG. The results are shown in Figure 1.

Experiment 2
Students mixed a PA concentration of 0.5 M in a 50 ml aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from propionic acid to 1-PrOH. The results are shown in Figure 2.

Q. Based on the data in Tables 1 and 2, which of the following best describes the results of the experiments?
As conversion time increased, the percent of acid converted:
As conversion time increased, the percent of acid converted:
- a)decreased at some temperatures only until conversion stopped.
- b)increased at some temperatures only until conversion stopped.
- c)decreased at all temperatures until conversion stopped.
- d)increased at all temperatures until conversion stopped.
Correct answer is option 'D'. Can you explain this answer?
Most Upvoted Answer
Directions:Read the passages and choose the best answer to each questi...
The data in Table 1 and Table 2 indicate a direct relationship between time and percentage of conversion. As time increased, at each temperature the percent of acid converted also increased up to a certain point, at which time conversion stopped.

|
Explore Courses for ACT exam
|

|
Directions:Read the passages and choose the best answer to each question.PassageStudents studied the effect of temperature on the conversion rates of two organic acids to their corresponding alcohols. The two organic acids studied were lactic acid (LA) and propionic acid (PA). Each acid was mixed with an Ru/C catalyst (to start the conversion) in an aqueous (water) solution. Lactic acid was found to break down into propylene glycol (PG), water, and various carbon side products. Propionic acid was found to break down into 1-propanol (1-PrOH), water, and various carbon side products. For all experiments, temperature was measured in degrees Kelvin (K).Experiment 1Students mixed an LA concentration of 0.5 moles (M) in a 50-milliliter (ml) aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from lactic acid to PG. The results are shown in Figure 1.Experiment 2Students mixed a PA concentration of 0.5 M in a 50 ml aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from propionic acid to 1-PrOH. The results are shown in Figure 2.Q.Based on the data in Tables 1 and 2, which of the following best describes the results of the experiments?As conversion time increased, the percent of acid converted:a)decreased at some temperatures only until conversion stopped.b)increased at some temperatures only until conversion stopped.c)decreased at all temperatures until conversion stopped.d)increased at all temperatures until conversion stopped.Correct answer is option 'D'. Can you explain this answer?
Question Description
Directions:Read the passages and choose the best answer to each question.PassageStudents studied the effect of temperature on the conversion rates of two organic acids to their corresponding alcohols. The two organic acids studied were lactic acid (LA) and propionic acid (PA). Each acid was mixed with an Ru/C catalyst (to start the conversion) in an aqueous (water) solution. Lactic acid was found to break down into propylene glycol (PG), water, and various carbon side products. Propionic acid was found to break down into 1-propanol (1-PrOH), water, and various carbon side products. For all experiments, temperature was measured in degrees Kelvin (K).Experiment 1Students mixed an LA concentration of 0.5 moles (M) in a 50-milliliter (ml) aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from lactic acid to PG. The results are shown in Figure 1.Experiment 2Students mixed a PA concentration of 0.5 M in a 50 ml aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from propionic acid to 1-PrOH. The results are shown in Figure 2.Q.Based on the data in Tables 1 and 2, which of the following best describes the results of the experiments?As conversion time increased, the percent of acid converted:a)decreased at some temperatures only until conversion stopped.b)increased at some temperatures only until conversion stopped.c)decreased at all temperatures until conversion stopped.d)increased at all temperatures until conversion stopped.Correct answer is option 'D'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Directions:Read the passages and choose the best answer to each question.PassageStudents studied the effect of temperature on the conversion rates of two organic acids to their corresponding alcohols. The two organic acids studied were lactic acid (LA) and propionic acid (PA). Each acid was mixed with an Ru/C catalyst (to start the conversion) in an aqueous (water) solution. Lactic acid was found to break down into propylene glycol (PG), water, and various carbon side products. Propionic acid was found to break down into 1-propanol (1-PrOH), water, and various carbon side products. For all experiments, temperature was measured in degrees Kelvin (K).Experiment 1Students mixed an LA concentration of 0.5 moles (M) in a 50-milliliter (ml) aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from lactic acid to PG. The results are shown in Figure 1.Experiment 2Students mixed a PA concentration of 0.5 M in a 50 ml aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from propionic acid to 1-PrOH. The results are shown in Figure 2.Q.Based on the data in Tables 1 and 2, which of the following best describes the results of the experiments?As conversion time increased, the percent of acid converted:a)decreased at some temperatures only until conversion stopped.b)increased at some temperatures only until conversion stopped.c)decreased at all temperatures until conversion stopped.d)increased at all temperatures until conversion stopped.Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions:Read the passages and choose the best answer to each question.PassageStudents studied the effect of temperature on the conversion rates of two organic acids to their corresponding alcohols. The two organic acids studied were lactic acid (LA) and propionic acid (PA). Each acid was mixed with an Ru/C catalyst (to start the conversion) in an aqueous (water) solution. Lactic acid was found to break down into propylene glycol (PG), water, and various carbon side products. Propionic acid was found to break down into 1-propanol (1-PrOH), water, and various carbon side products. For all experiments, temperature was measured in degrees Kelvin (K).Experiment 1Students mixed an LA concentration of 0.5 moles (M) in a 50-milliliter (ml) aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from lactic acid to PG. The results are shown in Figure 1.Experiment 2Students mixed a PA concentration of 0.5 M in a 50 ml aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from propionic acid to 1-PrOH. The results are shown in Figure 2.Q.Based on the data in Tables 1 and 2, which of the following best describes the results of the experiments?As conversion time increased, the percent of acid converted:a)decreased at some temperatures only until conversion stopped.b)increased at some temperatures only until conversion stopped.c)decreased at all temperatures until conversion stopped.d)increased at all temperatures until conversion stopped.Correct answer is option 'D'. Can you explain this answer?.
Directions:Read the passages and choose the best answer to each question.PassageStudents studied the effect of temperature on the conversion rates of two organic acids to their corresponding alcohols. The two organic acids studied were lactic acid (LA) and propionic acid (PA). Each acid was mixed with an Ru/C catalyst (to start the conversion) in an aqueous (water) solution. Lactic acid was found to break down into propylene glycol (PG), water, and various carbon side products. Propionic acid was found to break down into 1-propanol (1-PrOH), water, and various carbon side products. For all experiments, temperature was measured in degrees Kelvin (K).Experiment 1Students mixed an LA concentration of 0.5 moles (M) in a 50-milliliter (ml) aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from lactic acid to PG. The results are shown in Figure 1.Experiment 2Students mixed a PA concentration of 0.5 M in a 50 ml aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from propionic acid to 1-PrOH. The results are shown in Figure 2.Q.Based on the data in Tables 1 and 2, which of the following best describes the results of the experiments?As conversion time increased, the percent of acid converted:a)decreased at some temperatures only until conversion stopped.b)increased at some temperatures only until conversion stopped.c)decreased at all temperatures until conversion stopped.d)increased at all temperatures until conversion stopped.Correct answer is option 'D'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Directions:Read the passages and choose the best answer to each question.PassageStudents studied the effect of temperature on the conversion rates of two organic acids to their corresponding alcohols. The two organic acids studied were lactic acid (LA) and propionic acid (PA). Each acid was mixed with an Ru/C catalyst (to start the conversion) in an aqueous (water) solution. Lactic acid was found to break down into propylene glycol (PG), water, and various carbon side products. Propionic acid was found to break down into 1-propanol (1-PrOH), water, and various carbon side products. For all experiments, temperature was measured in degrees Kelvin (K).Experiment 1Students mixed an LA concentration of 0.5 moles (M) in a 50-milliliter (ml) aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from lactic acid to PG. The results are shown in Figure 1.Experiment 2Students mixed a PA concentration of 0.5 M in a 50 ml aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from propionic acid to 1-PrOH. The results are shown in Figure 2.Q.Based on the data in Tables 1 and 2, which of the following best describes the results of the experiments?As conversion time increased, the percent of acid converted:a)decreased at some temperatures only until conversion stopped.b)increased at some temperatures only until conversion stopped.c)decreased at all temperatures until conversion stopped.d)increased at all temperatures until conversion stopped.Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions:Read the passages and choose the best answer to each question.PassageStudents studied the effect of temperature on the conversion rates of two organic acids to their corresponding alcohols. The two organic acids studied were lactic acid (LA) and propionic acid (PA). Each acid was mixed with an Ru/C catalyst (to start the conversion) in an aqueous (water) solution. Lactic acid was found to break down into propylene glycol (PG), water, and various carbon side products. Propionic acid was found to break down into 1-propanol (1-PrOH), water, and various carbon side products. For all experiments, temperature was measured in degrees Kelvin (K).Experiment 1Students mixed an LA concentration of 0.5 moles (M) in a 50-milliliter (ml) aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from lactic acid to PG. The results are shown in Figure 1.Experiment 2Students mixed a PA concentration of 0.5 M in a 50 ml aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from propionic acid to 1-PrOH. The results are shown in Figure 2.Q.Based on the data in Tables 1 and 2, which of the following best describes the results of the experiments?As conversion time increased, the percent of acid converted:a)decreased at some temperatures only until conversion stopped.b)increased at some temperatures only until conversion stopped.c)decreased at all temperatures until conversion stopped.d)increased at all temperatures until conversion stopped.Correct answer is option 'D'. Can you explain this answer?.
Solutions for Directions:Read the passages and choose the best answer to each question.PassageStudents studied the effect of temperature on the conversion rates of two organic acids to their corresponding alcohols. The two organic acids studied were lactic acid (LA) and propionic acid (PA). Each acid was mixed with an Ru/C catalyst (to start the conversion) in an aqueous (water) solution. Lactic acid was found to break down into propylene glycol (PG), water, and various carbon side products. Propionic acid was found to break down into 1-propanol (1-PrOH), water, and various carbon side products. For all experiments, temperature was measured in degrees Kelvin (K).Experiment 1Students mixed an LA concentration of 0.5 moles (M) in a 50-milliliter (ml) aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from lactic acid to PG. The results are shown in Figure 1.Experiment 2Students mixed a PA concentration of 0.5 M in a 50 ml aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from propionic acid to 1-PrOH. The results are shown in Figure 2.Q.Based on the data in Tables 1 and 2, which of the following best describes the results of the experiments?As conversion time increased, the percent of acid converted:a)decreased at some temperatures only until conversion stopped.b)increased at some temperatures only until conversion stopped.c)decreased at all temperatures until conversion stopped.d)increased at all temperatures until conversion stopped.Correct answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for ACT.
Download more important topics, notes, lectures and mock test series for ACT Exam by signing up for free.
Here you can find the meaning of Directions:Read the passages and choose the best answer to each question.PassageStudents studied the effect of temperature on the conversion rates of two organic acids to their corresponding alcohols. The two organic acids studied were lactic acid (LA) and propionic acid (PA). Each acid was mixed with an Ru/C catalyst (to start the conversion) in an aqueous (water) solution. Lactic acid was found to break down into propylene glycol (PG), water, and various carbon side products. Propionic acid was found to break down into 1-propanol (1-PrOH), water, and various carbon side products. For all experiments, temperature was measured in degrees Kelvin (K).Experiment 1Students mixed an LA concentration of 0.5 moles (M) in a 50-milliliter (ml) aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from lactic acid to PG. The results are shown in Figure 1.Experiment 2Students mixed a PA concentration of 0.5 M in a 50 ml aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from propionic acid to 1-PrOH. The results are shown in Figure 2.Q.Based on the data in Tables 1 and 2, which of the following best describes the results of the experiments?As conversion time increased, the percent of acid converted:a)decreased at some temperatures only until conversion stopped.b)increased at some temperatures only until conversion stopped.c)decreased at all temperatures until conversion stopped.d)increased at all temperatures until conversion stopped.Correct answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Directions:Read the passages and choose the best answer to each question.PassageStudents studied the effect of temperature on the conversion rates of two organic acids to their corresponding alcohols. The two organic acids studied were lactic acid (LA) and propionic acid (PA). Each acid was mixed with an Ru/C catalyst (to start the conversion) in an aqueous (water) solution. Lactic acid was found to break down into propylene glycol (PG), water, and various carbon side products. Propionic acid was found to break down into 1-propanol (1-PrOH), water, and various carbon side products. For all experiments, temperature was measured in degrees Kelvin (K).Experiment 1Students mixed an LA concentration of 0.5 moles (M) in a 50-milliliter (ml) aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from lactic acid to PG. The results are shown in Figure 1.Experiment 2Students mixed a PA concentration of 0.5 M in a 50 ml aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from propionic acid to 1-PrOH. The results are shown in Figure 2.Q.Based on the data in Tables 1 and 2, which of the following best describes the results of the experiments?As conversion time increased, the percent of acid converted:a)decreased at some temperatures only until conversion stopped.b)increased at some temperatures only until conversion stopped.c)decreased at all temperatures until conversion stopped.d)increased at all temperatures until conversion stopped.Correct answer is option 'D'. Can you explain this answer?, a detailed solution for Directions:Read the passages and choose the best answer to each question.PassageStudents studied the effect of temperature on the conversion rates of two organic acids to their corresponding alcohols. The two organic acids studied were lactic acid (LA) and propionic acid (PA). Each acid was mixed with an Ru/C catalyst (to start the conversion) in an aqueous (water) solution. Lactic acid was found to break down into propylene glycol (PG), water, and various carbon side products. Propionic acid was found to break down into 1-propanol (1-PrOH), water, and various carbon side products. For all experiments, temperature was measured in degrees Kelvin (K).Experiment 1Students mixed an LA concentration of 0.5 moles (M) in a 50-milliliter (ml) aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from lactic acid to PG. The results are shown in Figure 1.Experiment 2Students mixed a PA concentration of 0.5 M in a 50 ml aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from propionic acid to 1-PrOH. The results are shown in Figure 2.Q.Based on the data in Tables 1 and 2, which of the following best describes the results of the experiments?As conversion time increased, the percent of acid converted:a)decreased at some temperatures only until conversion stopped.b)increased at some temperatures only until conversion stopped.c)decreased at all temperatures until conversion stopped.d)increased at all temperatures until conversion stopped.Correct answer is option 'D'. Can you explain this answer? has been provided alongside types of Directions:Read the passages and choose the best answer to each question.PassageStudents studied the effect of temperature on the conversion rates of two organic acids to their corresponding alcohols. The two organic acids studied were lactic acid (LA) and propionic acid (PA). Each acid was mixed with an Ru/C catalyst (to start the conversion) in an aqueous (water) solution. Lactic acid was found to break down into propylene glycol (PG), water, and various carbon side products. Propionic acid was found to break down into 1-propanol (1-PrOH), water, and various carbon side products. For all experiments, temperature was measured in degrees Kelvin (K).Experiment 1Students mixed an LA concentration of 0.5 moles (M) in a 50-milliliter (ml) aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from lactic acid to PG. The results are shown in Figure 1.Experiment 2Students mixed a PA concentration of 0.5 M in a 50 ml aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from propionic acid to 1-PrOH. The results are shown in Figure 2.Q.Based on the data in Tables 1 and 2, which of the following best describes the results of the experiments?As conversion time increased, the percent of acid converted:a)decreased at some temperatures only until conversion stopped.b)increased at some temperatures only until conversion stopped.c)decreased at all temperatures until conversion stopped.d)increased at all temperatures until conversion stopped.Correct answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Directions:Read the passages and choose the best answer to each question.PassageStudents studied the effect of temperature on the conversion rates of two organic acids to their corresponding alcohols. The two organic acids studied were lactic acid (LA) and propionic acid (PA). Each acid was mixed with an Ru/C catalyst (to start the conversion) in an aqueous (water) solution. Lactic acid was found to break down into propylene glycol (PG), water, and various carbon side products. Propionic acid was found to break down into 1-propanol (1-PrOH), water, and various carbon side products. For all experiments, temperature was measured in degrees Kelvin (K).Experiment 1Students mixed an LA concentration of 0.5 moles (M) in a 50-milliliter (ml) aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from lactic acid to PG. The results are shown in Figure 1.Experiment 2Students mixed a PA concentration of 0.5 M in a 50 ml aqueous solution along with a 5% Ru/C catalyst. The temperature was then varied to study the effect on the rate of conversion from propionic acid to 1-PrOH. The results are shown in Figure 2.Q.Based on the data in Tables 1 and 2, which of the following best describes the results of the experiments?As conversion time increased, the percent of acid converted:a)decreased at some temperatures only until conversion stopped.b)increased at some temperatures only until conversion stopped.c)decreased at all temperatures until conversion stopped.d)increased at all temperatures until conversion stopped.Correct answer is option 'D'. Can you explain this answer? tests, examples and also practice ACT tests.

|
Explore Courses for ACT exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























