MCAT Exam > MCAT Questions > Which of the following statements about the m...
Start Learning for Free
Which of the following statements about the melting and boiling points of the organic compounds can be deduced most accurately represent the data below?
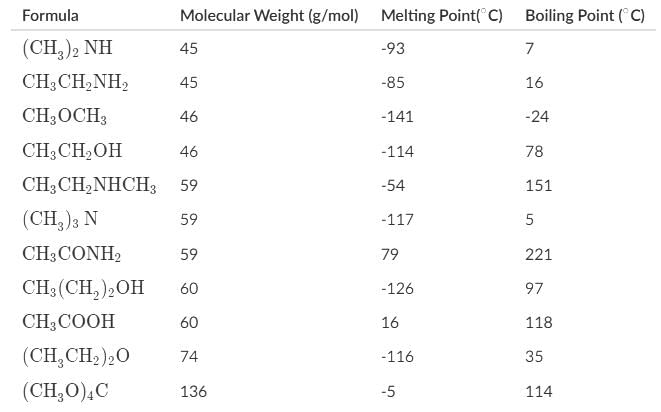
- a)The dimeric H-bonded structure appears to be a good representation of acetic acid in the gaseous state based on the molecular weight and boiling point of tetramethoxymethane.
- b)Carboxylic acids generally have the highest boiling points of the organic compounds due to their ability to dimerize in the liquid phase.
- c)Most amides are solids at room temperature due to such high melting points, and for 1° and 2° amides, their high boiling points are attributed to the hydrogen bonding, and for 3° amides, to their high polarity.
- d)Alcohols, carboxylic acids, amines, and amides may serve as hydrogen-bond donors and acceptors, which accounts for their higher boiling points in comparison to ethers and ketones.
Correct answer is option 'C'. Can you explain this answer?
Verified Answer
Which of the following statements about the melting and boiling points...
Alcohols, carboxylic acids, and only 1° and 2° amines and amides may serve as H-bond donors and acceptors. Ethers, ketones, 3° amines and amides can serve as acceptors.
Carboxylic acids generally have high boiling points, but amides can technically have boiling points almost 100 degrees greater than carboxylic acids
The dimeric H-bonded structure appears to be a good representation of acetic acid in both the gaseous and liquid state. If acetic acid dimerized, it would have a molecular weight of 120 and a boiling point similar to a molecule of that weight.
Tetramethoxymethane is a molecule with a molecular weight of 136, which is sufficiently close to 120, and a boiling point of 114. The similar boiling point with acetic acid confirms our suspicions that it dimerized in liquid and gaseous phases.
Amides indeed have high melting points, which makes most of them solids at room temperatures. Amides have higher boiling points than carboxylic acids due to its highly polar carbonyl group as indicated by its resonance structure, leaving carbon with a negative charge and nitrogen with a positive charge, and the H-bonding between primary and secondary amides.
Most Upvoted Answer
Which of the following statements about the melting and boiling points...
Alcohols, carboxylic acids, and only 1° and 2° amines and amides may serve as H-bond donors and acceptors. Ethers, ketones, 3° amines and amides can serve as acceptors.
Carboxylic acids generally have high boiling points, but amides can technically have boiling points almost 100 degrees greater than carboxylic acids
The dimeric H-bonded structure appears to be a good representation of acetic acid in both the gaseous and liquid state. If acetic acid dimerized, it would have a molecular weight of 120 and a boiling point similar to a molecule of that weight.
Tetramethoxymethane is a molecule with a molecular weight of 136, which is sufficiently close to 120, and a boiling point of 114. The similar boiling point with acetic acid confirms our suspicions that it dimerized in liquid and gaseous phases.
Amides indeed have high melting points, which makes most of them solids at room temperatures. Amides have higher boiling points than carboxylic acids due to its highly polar carbonyl group as indicated by its resonance structure, leaving carbon with a negative charge and nitrogen with a positive charge, and the H-bonding between primary and secondary amides.

|
Explore Courses for MCAT exam
|

|
Question Description
Which of the following statements about the melting and boiling points of the organic compounds can be deduced most accurately represent the data below?a)The dimeric H-bonded structure appears to be a good representation of acetic acid in the gaseous state based on the molecular weight and boiling point of tetramethoxymethane.b)Carboxylic acids generally have the highest boiling points of the organic compounds due to their ability to dimerize in the liquid phase.c)Most amides are solids at room temperature due to such high melting points, and for 1° and 2° amides, their high boiling points are attributed to the hydrogen bonding, and for 3° amides, to their high polarity.d)Alcohols, carboxylic acids, amines, and amides may serve as hydrogen-bond donors and acceptors, which accounts for their higher boiling points in comparison to ethers and ketones.Correct answer is option 'C'. Can you explain this answer? for MCAT 2025 is part of MCAT preparation. The Question and answers have been prepared according to the MCAT exam syllabus. Information about Which of the following statements about the melting and boiling points of the organic compounds can be deduced most accurately represent the data below?a)The dimeric H-bonded structure appears to be a good representation of acetic acid in the gaseous state based on the molecular weight and boiling point of tetramethoxymethane.b)Carboxylic acids generally have the highest boiling points of the organic compounds due to their ability to dimerize in the liquid phase.c)Most amides are solids at room temperature due to such high melting points, and for 1° and 2° amides, their high boiling points are attributed to the hydrogen bonding, and for 3° amides, to their high polarity.d)Alcohols, carboxylic acids, amines, and amides may serve as hydrogen-bond donors and acceptors, which accounts for their higher boiling points in comparison to ethers and ketones.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for MCAT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following statements about the melting and boiling points of the organic compounds can be deduced most accurately represent the data below?a)The dimeric H-bonded structure appears to be a good representation of acetic acid in the gaseous state based on the molecular weight and boiling point of tetramethoxymethane.b)Carboxylic acids generally have the highest boiling points of the organic compounds due to their ability to dimerize in the liquid phase.c)Most amides are solids at room temperature due to such high melting points, and for 1° and 2° amides, their high boiling points are attributed to the hydrogen bonding, and for 3° amides, to their high polarity.d)Alcohols, carboxylic acids, amines, and amides may serve as hydrogen-bond donors and acceptors, which accounts for their higher boiling points in comparison to ethers and ketones.Correct answer is option 'C'. Can you explain this answer?.
Which of the following statements about the melting and boiling points of the organic compounds can be deduced most accurately represent the data below?a)The dimeric H-bonded structure appears to be a good representation of acetic acid in the gaseous state based on the molecular weight and boiling point of tetramethoxymethane.b)Carboxylic acids generally have the highest boiling points of the organic compounds due to their ability to dimerize in the liquid phase.c)Most amides are solids at room temperature due to such high melting points, and for 1° and 2° amides, their high boiling points are attributed to the hydrogen bonding, and for 3° amides, to their high polarity.d)Alcohols, carboxylic acids, amines, and amides may serve as hydrogen-bond donors and acceptors, which accounts for their higher boiling points in comparison to ethers and ketones.Correct answer is option 'C'. Can you explain this answer? for MCAT 2025 is part of MCAT preparation. The Question and answers have been prepared according to the MCAT exam syllabus. Information about Which of the following statements about the melting and boiling points of the organic compounds can be deduced most accurately represent the data below?a)The dimeric H-bonded structure appears to be a good representation of acetic acid in the gaseous state based on the molecular weight and boiling point of tetramethoxymethane.b)Carboxylic acids generally have the highest boiling points of the organic compounds due to their ability to dimerize in the liquid phase.c)Most amides are solids at room temperature due to such high melting points, and for 1° and 2° amides, their high boiling points are attributed to the hydrogen bonding, and for 3° amides, to their high polarity.d)Alcohols, carboxylic acids, amines, and amides may serve as hydrogen-bond donors and acceptors, which accounts for their higher boiling points in comparison to ethers and ketones.Correct answer is option 'C'. Can you explain this answer? covers all topics & solutions for MCAT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following statements about the melting and boiling points of the organic compounds can be deduced most accurately represent the data below?a)The dimeric H-bonded structure appears to be a good representation of acetic acid in the gaseous state based on the molecular weight and boiling point of tetramethoxymethane.b)Carboxylic acids generally have the highest boiling points of the organic compounds due to their ability to dimerize in the liquid phase.c)Most amides are solids at room temperature due to such high melting points, and for 1° and 2° amides, their high boiling points are attributed to the hydrogen bonding, and for 3° amides, to their high polarity.d)Alcohols, carboxylic acids, amines, and amides may serve as hydrogen-bond donors and acceptors, which accounts for their higher boiling points in comparison to ethers and ketones.Correct answer is option 'C'. Can you explain this answer?.
Solutions for Which of the following statements about the melting and boiling points of the organic compounds can be deduced most accurately represent the data below?a)The dimeric H-bonded structure appears to be a good representation of acetic acid in the gaseous state based on the molecular weight and boiling point of tetramethoxymethane.b)Carboxylic acids generally have the highest boiling points of the organic compounds due to their ability to dimerize in the liquid phase.c)Most amides are solids at room temperature due to such high melting points, and for 1° and 2° amides, their high boiling points are attributed to the hydrogen bonding, and for 3° amides, to their high polarity.d)Alcohols, carboxylic acids, amines, and amides may serve as hydrogen-bond donors and acceptors, which accounts for their higher boiling points in comparison to ethers and ketones.Correct answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for MCAT.
Download more important topics, notes, lectures and mock test series for MCAT Exam by signing up for free.
Here you can find the meaning of Which of the following statements about the melting and boiling points of the organic compounds can be deduced most accurately represent the data below?a)The dimeric H-bonded structure appears to be a good representation of acetic acid in the gaseous state based on the molecular weight and boiling point of tetramethoxymethane.b)Carboxylic acids generally have the highest boiling points of the organic compounds due to their ability to dimerize in the liquid phase.c)Most amides are solids at room temperature due to such high melting points, and for 1° and 2° amides, their high boiling points are attributed to the hydrogen bonding, and for 3° amides, to their high polarity.d)Alcohols, carboxylic acids, amines, and amides may serve as hydrogen-bond donors and acceptors, which accounts for their higher boiling points in comparison to ethers and ketones.Correct answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following statements about the melting and boiling points of the organic compounds can be deduced most accurately represent the data below?a)The dimeric H-bonded structure appears to be a good representation of acetic acid in the gaseous state based on the molecular weight and boiling point of tetramethoxymethane.b)Carboxylic acids generally have the highest boiling points of the organic compounds due to their ability to dimerize in the liquid phase.c)Most amides are solids at room temperature due to such high melting points, and for 1° and 2° amides, their high boiling points are attributed to the hydrogen bonding, and for 3° amides, to their high polarity.d)Alcohols, carboxylic acids, amines, and amides may serve as hydrogen-bond donors and acceptors, which accounts for their higher boiling points in comparison to ethers and ketones.Correct answer is option 'C'. Can you explain this answer?, a detailed solution for Which of the following statements about the melting and boiling points of the organic compounds can be deduced most accurately represent the data below?a)The dimeric H-bonded structure appears to be a good representation of acetic acid in the gaseous state based on the molecular weight and boiling point of tetramethoxymethane.b)Carboxylic acids generally have the highest boiling points of the organic compounds due to their ability to dimerize in the liquid phase.c)Most amides are solids at room temperature due to such high melting points, and for 1° and 2° amides, their high boiling points are attributed to the hydrogen bonding, and for 3° amides, to their high polarity.d)Alcohols, carboxylic acids, amines, and amides may serve as hydrogen-bond donors and acceptors, which accounts for their higher boiling points in comparison to ethers and ketones.Correct answer is option 'C'. Can you explain this answer? has been provided alongside types of Which of the following statements about the melting and boiling points of the organic compounds can be deduced most accurately represent the data below?a)The dimeric H-bonded structure appears to be a good representation of acetic acid in the gaseous state based on the molecular weight and boiling point of tetramethoxymethane.b)Carboxylic acids generally have the highest boiling points of the organic compounds due to their ability to dimerize in the liquid phase.c)Most amides are solids at room temperature due to such high melting points, and for 1° and 2° amides, their high boiling points are attributed to the hydrogen bonding, and for 3° amides, to their high polarity.d)Alcohols, carboxylic acids, amines, and amides may serve as hydrogen-bond donors and acceptors, which accounts for their higher boiling points in comparison to ethers and ketones.Correct answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following statements about the melting and boiling points of the organic compounds can be deduced most accurately represent the data below?a)The dimeric H-bonded structure appears to be a good representation of acetic acid in the gaseous state based on the molecular weight and boiling point of tetramethoxymethane.b)Carboxylic acids generally have the highest boiling points of the organic compounds due to their ability to dimerize in the liquid phase.c)Most amides are solids at room temperature due to such high melting points, and for 1° and 2° amides, their high boiling points are attributed to the hydrogen bonding, and for 3° amides, to their high polarity.d)Alcohols, carboxylic acids, amines, and amides may serve as hydrogen-bond donors and acceptors, which accounts for their higher boiling points in comparison to ethers and ketones.Correct answer is option 'C'. Can you explain this answer? tests, examples and also practice MCAT tests.

|
Explore Courses for MCAT exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















