ACT Exam > ACT Questions > Directions:Read the passage and choose the be...
Start Learning for Free
Directions: Read the passage and choose the best answer to each question.
Passage
Petroleum, or crude oil, is refined by separating it into different by-products. This process is called fractional distillation, whereby the crude oil is heated and each different product is distilled, or drawn off, at different stages. Each product is distilled at certain temperature ranges and collected in separate receivers. Petroleum refining is carried out in a boiler and a fractionating tower. The crude oil is super-heated in the boiler to about 600◦ C, which vaporizes the crude oil. The vapors then rise in the tower to certain levels where they cool and condense, according to their chemical structure. When the vapor reaches a height in the tower where the temperature in the column is equal to the boiling point of the substance, the vapor turns into liquid (condenses), collects in troughs, and flows into various tanks for storage, as shown in Figure 1. Table 1 below summarizes the characteristics of the by-products obtained from the fractional distillation of petroleum.
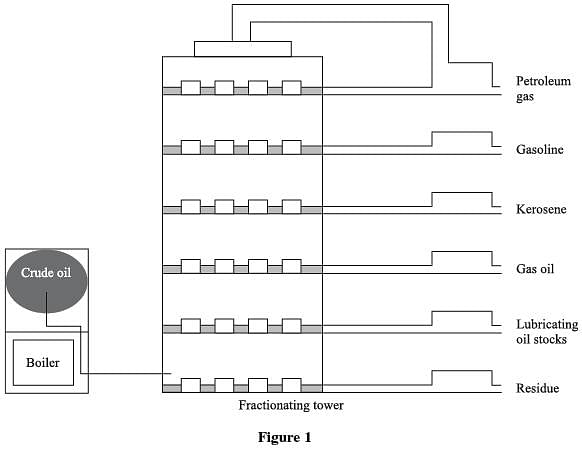
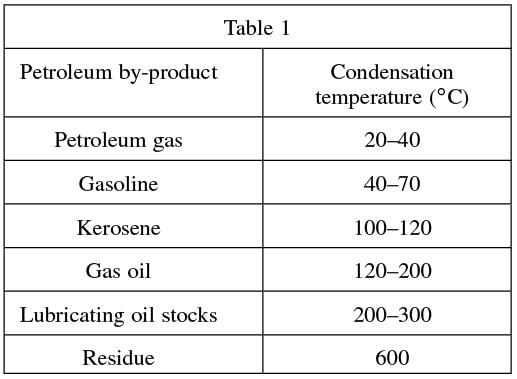
Passage
Petroleum, or crude oil, is refined by separating it into different by-products. This process is called fractional distillation, whereby the crude oil is heated and each different product is distilled, or drawn off, at different stages. Each product is distilled at certain temperature ranges and collected in separate receivers. Petroleum refining is carried out in a boiler and a fractionating tower. The crude oil is super-heated in the boiler to about 600◦ C, which vaporizes the crude oil. The vapors then rise in the tower to certain levels where they cool and condense, according to their chemical structure. When the vapor reaches a height in the tower where the temperature in the column is equal to the boiling point of the substance, the vapor turns into liquid (condenses), collects in troughs, and flows into various tanks for storage, as shown in Figure 1. Table 1 below summarizes the characteristics of the by-products obtained from the fractional distillation of petroleum.


Q. Given that naptha, another by-product of petroleum distillation, has a condensation point of approximately 90◦C, between which two petroleum by-products would this substance be found in a fractionating tower?
- a)Gasoline and kerosene
- b)Lubricating oil stocks and gas oil
- c)Kerosene and gas oil
- d)Residue and lubricating oil stocks
Correct answer is option 'A'. Can you explain this answer?
Most Upvoted Answer
Directions:Read the passage and choose the best answer to each questio...
The best answer is a. A condensation point of 90◦C would place naptha in Table 1 between gasoline (40–70◦C) and kerosene (100–120◦C), as it is above the upper end of gasoline and below the lower end of kerosene.

|
Explore Courses for ACT exam
|

|
Directions:Read the passage and choose the best answer to each question.PassagePetroleum, or crude oil, is refined by separating it into different by-products. This process is called fractional distillation, whereby the crude oil is heated and each different product is distilled, or drawn off, at different stages. Each product is distilled at certain temperature ranges and collected in separate receivers. Petroleum refining is carried out in a boiler and a fractionating tower. The crude oil is super-heated in the boiler to about 600 C, which vaporizes the crude oil. The vapors then rise in the tower to certain levels where they cool and condense, according to their chemical structure. When the vapor reaches a height in the tower where the temperature in the column is equal to the boiling point of the substance, the vapor turns into liquid (condenses), collects in troughs, and flows into various tanks for storage, as shown in Figure 1. Table 1 below summarizes the characteristics of the by-products obtained from the fractional distillation of petroleum.Q. Given that naptha, another by-product of petroleum distillation, has a condensation point of approximately 90C, between which two petroleum by-products would this substance be found in a fractionating tower?a)Gasoline and keroseneb)Lubricating oil stocks and gas oilc)Kerosene and gas oild)Residue and lubricating oil stocksCorrect answer is option 'A'. Can you explain this answer?
Question Description
Directions:Read the passage and choose the best answer to each question.PassagePetroleum, or crude oil, is refined by separating it into different by-products. This process is called fractional distillation, whereby the crude oil is heated and each different product is distilled, or drawn off, at different stages. Each product is distilled at certain temperature ranges and collected in separate receivers. Petroleum refining is carried out in a boiler and a fractionating tower. The crude oil is super-heated in the boiler to about 600 C, which vaporizes the crude oil. The vapors then rise in the tower to certain levels where they cool and condense, according to their chemical structure. When the vapor reaches a height in the tower where the temperature in the column is equal to the boiling point of the substance, the vapor turns into liquid (condenses), collects in troughs, and flows into various tanks for storage, as shown in Figure 1. Table 1 below summarizes the characteristics of the by-products obtained from the fractional distillation of petroleum.Q. Given that naptha, another by-product of petroleum distillation, has a condensation point of approximately 90C, between which two petroleum by-products would this substance be found in a fractionating tower?a)Gasoline and keroseneb)Lubricating oil stocks and gas oilc)Kerosene and gas oild)Residue and lubricating oil stocksCorrect answer is option 'A'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Directions:Read the passage and choose the best answer to each question.PassagePetroleum, or crude oil, is refined by separating it into different by-products. This process is called fractional distillation, whereby the crude oil is heated and each different product is distilled, or drawn off, at different stages. Each product is distilled at certain temperature ranges and collected in separate receivers. Petroleum refining is carried out in a boiler and a fractionating tower. The crude oil is super-heated in the boiler to about 600 C, which vaporizes the crude oil. The vapors then rise in the tower to certain levels where they cool and condense, according to their chemical structure. When the vapor reaches a height in the tower where the temperature in the column is equal to the boiling point of the substance, the vapor turns into liquid (condenses), collects in troughs, and flows into various tanks for storage, as shown in Figure 1. Table 1 below summarizes the characteristics of the by-products obtained from the fractional distillation of petroleum.Q. Given that naptha, another by-product of petroleum distillation, has a condensation point of approximately 90C, between which two petroleum by-products would this substance be found in a fractionating tower?a)Gasoline and keroseneb)Lubricating oil stocks and gas oilc)Kerosene and gas oild)Residue and lubricating oil stocksCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions:Read the passage and choose the best answer to each question.PassagePetroleum, or crude oil, is refined by separating it into different by-products. This process is called fractional distillation, whereby the crude oil is heated and each different product is distilled, or drawn off, at different stages. Each product is distilled at certain temperature ranges and collected in separate receivers. Petroleum refining is carried out in a boiler and a fractionating tower. The crude oil is super-heated in the boiler to about 600 C, which vaporizes the crude oil. The vapors then rise in the tower to certain levels where they cool and condense, according to their chemical structure. When the vapor reaches a height in the tower where the temperature in the column is equal to the boiling point of the substance, the vapor turns into liquid (condenses), collects in troughs, and flows into various tanks for storage, as shown in Figure 1. Table 1 below summarizes the characteristics of the by-products obtained from the fractional distillation of petroleum.Q. Given that naptha, another by-product of petroleum distillation, has a condensation point of approximately 90C, between which two petroleum by-products would this substance be found in a fractionating tower?a)Gasoline and keroseneb)Lubricating oil stocks and gas oilc)Kerosene and gas oild)Residue and lubricating oil stocksCorrect answer is option 'A'. Can you explain this answer?.
Directions:Read the passage and choose the best answer to each question.PassagePetroleum, or crude oil, is refined by separating it into different by-products. This process is called fractional distillation, whereby the crude oil is heated and each different product is distilled, or drawn off, at different stages. Each product is distilled at certain temperature ranges and collected in separate receivers. Petroleum refining is carried out in a boiler and a fractionating tower. The crude oil is super-heated in the boiler to about 600 C, which vaporizes the crude oil. The vapors then rise in the tower to certain levels where they cool and condense, according to their chemical structure. When the vapor reaches a height in the tower where the temperature in the column is equal to the boiling point of the substance, the vapor turns into liquid (condenses), collects in troughs, and flows into various tanks for storage, as shown in Figure 1. Table 1 below summarizes the characteristics of the by-products obtained from the fractional distillation of petroleum.Q. Given that naptha, another by-product of petroleum distillation, has a condensation point of approximately 90C, between which two petroleum by-products would this substance be found in a fractionating tower?a)Gasoline and keroseneb)Lubricating oil stocks and gas oilc)Kerosene and gas oild)Residue and lubricating oil stocksCorrect answer is option 'A'. Can you explain this answer? for ACT 2025 is part of ACT preparation. The Question and answers have been prepared according to the ACT exam syllabus. Information about Directions:Read the passage and choose the best answer to each question.PassagePetroleum, or crude oil, is refined by separating it into different by-products. This process is called fractional distillation, whereby the crude oil is heated and each different product is distilled, or drawn off, at different stages. Each product is distilled at certain temperature ranges and collected in separate receivers. Petroleum refining is carried out in a boiler and a fractionating tower. The crude oil is super-heated in the boiler to about 600 C, which vaporizes the crude oil. The vapors then rise in the tower to certain levels where they cool and condense, according to their chemical structure. When the vapor reaches a height in the tower where the temperature in the column is equal to the boiling point of the substance, the vapor turns into liquid (condenses), collects in troughs, and flows into various tanks for storage, as shown in Figure 1. Table 1 below summarizes the characteristics of the by-products obtained from the fractional distillation of petroleum.Q. Given that naptha, another by-product of petroleum distillation, has a condensation point of approximately 90C, between which two petroleum by-products would this substance be found in a fractionating tower?a)Gasoline and keroseneb)Lubricating oil stocks and gas oilc)Kerosene and gas oild)Residue and lubricating oil stocksCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for ACT 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Directions:Read the passage and choose the best answer to each question.PassagePetroleum, or crude oil, is refined by separating it into different by-products. This process is called fractional distillation, whereby the crude oil is heated and each different product is distilled, or drawn off, at different stages. Each product is distilled at certain temperature ranges and collected in separate receivers. Petroleum refining is carried out in a boiler and a fractionating tower. The crude oil is super-heated in the boiler to about 600 C, which vaporizes the crude oil. The vapors then rise in the tower to certain levels where they cool and condense, according to their chemical structure. When the vapor reaches a height in the tower where the temperature in the column is equal to the boiling point of the substance, the vapor turns into liquid (condenses), collects in troughs, and flows into various tanks for storage, as shown in Figure 1. Table 1 below summarizes the characteristics of the by-products obtained from the fractional distillation of petroleum.Q. Given that naptha, another by-product of petroleum distillation, has a condensation point of approximately 90C, between which two petroleum by-products would this substance be found in a fractionating tower?a)Gasoline and keroseneb)Lubricating oil stocks and gas oilc)Kerosene and gas oild)Residue and lubricating oil stocksCorrect answer is option 'A'. Can you explain this answer?.
Solutions for Directions:Read the passage and choose the best answer to each question.PassagePetroleum, or crude oil, is refined by separating it into different by-products. This process is called fractional distillation, whereby the crude oil is heated and each different product is distilled, or drawn off, at different stages. Each product is distilled at certain temperature ranges and collected in separate receivers. Petroleum refining is carried out in a boiler and a fractionating tower. The crude oil is super-heated in the boiler to about 600 C, which vaporizes the crude oil. The vapors then rise in the tower to certain levels where they cool and condense, according to their chemical structure. When the vapor reaches a height in the tower where the temperature in the column is equal to the boiling point of the substance, the vapor turns into liquid (condenses), collects in troughs, and flows into various tanks for storage, as shown in Figure 1. Table 1 below summarizes the characteristics of the by-products obtained from the fractional distillation of petroleum.Q. Given that naptha, another by-product of petroleum distillation, has a condensation point of approximately 90C, between which two petroleum by-products would this substance be found in a fractionating tower?a)Gasoline and keroseneb)Lubricating oil stocks and gas oilc)Kerosene and gas oild)Residue and lubricating oil stocksCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for ACT.
Download more important topics, notes, lectures and mock test series for ACT Exam by signing up for free.
Here you can find the meaning of Directions:Read the passage and choose the best answer to each question.PassagePetroleum, or crude oil, is refined by separating it into different by-products. This process is called fractional distillation, whereby the crude oil is heated and each different product is distilled, or drawn off, at different stages. Each product is distilled at certain temperature ranges and collected in separate receivers. Petroleum refining is carried out in a boiler and a fractionating tower. The crude oil is super-heated in the boiler to about 600 C, which vaporizes the crude oil. The vapors then rise in the tower to certain levels where they cool and condense, according to their chemical structure. When the vapor reaches a height in the tower where the temperature in the column is equal to the boiling point of the substance, the vapor turns into liquid (condenses), collects in troughs, and flows into various tanks for storage, as shown in Figure 1. Table 1 below summarizes the characteristics of the by-products obtained from the fractional distillation of petroleum.Q. Given that naptha, another by-product of petroleum distillation, has a condensation point of approximately 90C, between which two petroleum by-products would this substance be found in a fractionating tower?a)Gasoline and keroseneb)Lubricating oil stocks and gas oilc)Kerosene and gas oild)Residue and lubricating oil stocksCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Directions:Read the passage and choose the best answer to each question.PassagePetroleum, or crude oil, is refined by separating it into different by-products. This process is called fractional distillation, whereby the crude oil is heated and each different product is distilled, or drawn off, at different stages. Each product is distilled at certain temperature ranges and collected in separate receivers. Petroleum refining is carried out in a boiler and a fractionating tower. The crude oil is super-heated in the boiler to about 600 C, which vaporizes the crude oil. The vapors then rise in the tower to certain levels where they cool and condense, according to their chemical structure. When the vapor reaches a height in the tower where the temperature in the column is equal to the boiling point of the substance, the vapor turns into liquid (condenses), collects in troughs, and flows into various tanks for storage, as shown in Figure 1. Table 1 below summarizes the characteristics of the by-products obtained from the fractional distillation of petroleum.Q. Given that naptha, another by-product of petroleum distillation, has a condensation point of approximately 90C, between which two petroleum by-products would this substance be found in a fractionating tower?a)Gasoline and keroseneb)Lubricating oil stocks and gas oilc)Kerosene and gas oild)Residue and lubricating oil stocksCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for Directions:Read the passage and choose the best answer to each question.PassagePetroleum, or crude oil, is refined by separating it into different by-products. This process is called fractional distillation, whereby the crude oil is heated and each different product is distilled, or drawn off, at different stages. Each product is distilled at certain temperature ranges and collected in separate receivers. Petroleum refining is carried out in a boiler and a fractionating tower. The crude oil is super-heated in the boiler to about 600 C, which vaporizes the crude oil. The vapors then rise in the tower to certain levels where they cool and condense, according to their chemical structure. When the vapor reaches a height in the tower where the temperature in the column is equal to the boiling point of the substance, the vapor turns into liquid (condenses), collects in troughs, and flows into various tanks for storage, as shown in Figure 1. Table 1 below summarizes the characteristics of the by-products obtained from the fractional distillation of petroleum.Q. Given that naptha, another by-product of petroleum distillation, has a condensation point of approximately 90C, between which two petroleum by-products would this substance be found in a fractionating tower?a)Gasoline and keroseneb)Lubricating oil stocks and gas oilc)Kerosene and gas oild)Residue and lubricating oil stocksCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of Directions:Read the passage and choose the best answer to each question.PassagePetroleum, or crude oil, is refined by separating it into different by-products. This process is called fractional distillation, whereby the crude oil is heated and each different product is distilled, or drawn off, at different stages. Each product is distilled at certain temperature ranges and collected in separate receivers. Petroleum refining is carried out in a boiler and a fractionating tower. The crude oil is super-heated in the boiler to about 600 C, which vaporizes the crude oil. The vapors then rise in the tower to certain levels where they cool and condense, according to their chemical structure. When the vapor reaches a height in the tower where the temperature in the column is equal to the boiling point of the substance, the vapor turns into liquid (condenses), collects in troughs, and flows into various tanks for storage, as shown in Figure 1. Table 1 below summarizes the characteristics of the by-products obtained from the fractional distillation of petroleum.Q. Given that naptha, another by-product of petroleum distillation, has a condensation point of approximately 90C, between which two petroleum by-products would this substance be found in a fractionating tower?a)Gasoline and keroseneb)Lubricating oil stocks and gas oilc)Kerosene and gas oild)Residue and lubricating oil stocksCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Directions:Read the passage and choose the best answer to each question.PassagePetroleum, or crude oil, is refined by separating it into different by-products. This process is called fractional distillation, whereby the crude oil is heated and each different product is distilled, or drawn off, at different stages. Each product is distilled at certain temperature ranges and collected in separate receivers. Petroleum refining is carried out in a boiler and a fractionating tower. The crude oil is super-heated in the boiler to about 600 C, which vaporizes the crude oil. The vapors then rise in the tower to certain levels where they cool and condense, according to their chemical structure. When the vapor reaches a height in the tower where the temperature in the column is equal to the boiling point of the substance, the vapor turns into liquid (condenses), collects in troughs, and flows into various tanks for storage, as shown in Figure 1. Table 1 below summarizes the characteristics of the by-products obtained from the fractional distillation of petroleum.Q. Given that naptha, another by-product of petroleum distillation, has a condensation point of approximately 90C, between which two petroleum by-products would this substance be found in a fractionating tower?a)Gasoline and keroseneb)Lubricating oil stocks and gas oilc)Kerosene and gas oild)Residue and lubricating oil stocksCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice ACT tests.

|
Explore Courses for ACT exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























